| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:21:14 UTC |
|---|
| Update Date | 2020-04-22 15:17:11 UTC |
|---|
| BMDB ID | BMDB0006284 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
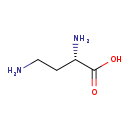
| Common Name | L-2,4-diaminobutyric acid |
|---|
| Description | L-2,4-diaminobutyric acid, also known as Dbu or alpha,gamma-diaminobutyrate, belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. Based on a literature review a significant number of articles have been published on L-2,4-diaminobutyric acid. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (S)-2,4-Diaminobutanoic acid | ChEBI | | alpha,gamma-Diaminobutyrate | ChEBI | | Dbu | ChEBI | | L-2,4-Diaminobutanoate | ChEBI | | L-2,4-Diaminobutanoic acid | ChEBI | | L-2,4-Diaminobutyrate | ChEBI | | L-Dbu | ChEBI | | L-Diaminobutyric acid | ChEBI | | (S)-2,4-Diaminobutanoate | Generator | | a,g-Diaminobutyrate | Generator | | a,g-Diaminobutyric acid | Generator | | alpha,gamma-Diaminobutyric acid | Generator | | Α,γ-diaminobutyrate | Generator | | Α,γ-diaminobutyric acid | Generator | | L-Diaminobutyrate | Generator | | (S)-2,4-diamino-Butanoate | HMDB | | (S)-2,4-diamino-Butanoic acid | HMDB | | (S)-2,4-Diaminobutyric acid | HMDB | | L-2,4-diamino-Butyric acid | HMDB | | L-2,4-diamino-N-Butyric acid | HMDB | | L-alpha,gamma-Diaminobutyric acid | HMDB | | L-DABA | HMDB | | 2,4-Diaminobutyric acid dihydrochloride, (+-)-isomer | MeSH, HMDB | | 2,4-Diaminobutyric acid monohydrochloride, (+-)-isomer | MeSH, HMDB | | 2,4-Diaminobutyric acid, (+)-isomer | MeSH, HMDB | | 2,4-Diaminobutyric acid, (+-)-isomer | MeSH, HMDB | | 2,4-Diaminobutyric acid, (R)-isomer | MeSH, HMDB | | 2,4-Diaminobutyric acid, (S)-isomer | MeSH, HMDB | | 2,4-Diaminobutyric acid dihydrochloride, (S)-isomer | MeSH, HMDB | | 2,4-Diaminobutyric acid | MeSH, HMDB | | 2,4-Diaminobutyric acid monohydrochloride, (S)-isomer | MeSH, HMDB |
|
|---|
| Chemical Formula | C4H10N2O2 |
|---|
| Average Molecular Weight | 118.1344 |
|---|
| Monoisotopic Molecular Weight | 118.074227574 |
|---|
| IUPAC Name | (2S)-2,4-diaminobutanoic acid |
|---|
| Traditional Name | 2,4-diaminobutyric acid |
|---|
| CAS Registry Number | 1758-80-1 |
|---|
| SMILES | NCC[C@H](N)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C4H10N2O2/c5-2-1-3(6)4(7)8/h3H,1-2,5-6H2,(H,7,8)/t3-/m0/s1 |
|---|
| InChI Key | OGNSCSPNOLGXSM-VKHMYHEASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | L-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - L-alpha-amino acid
- Amino fatty acid
- Fatty acid
- Fatty acyl
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Amine
- Hydrocarbon derivative
- Organic oxide
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Primary aliphatic amine
- Organic oxygen compound
- Carbonyl group
- Organic nitrogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (3 TMS) | splash10-0udi-0910000000-c063d8231376ede53de2 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (5 TMS) | splash10-0fe0-2910000000-db971ca13fbd6bdfb5f2 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (4 TMS) | splash10-0fki-1930000000-f3933f38127537d9d178 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0udi-0910000000-c063d8231376ede53de2 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0fe0-2910000000-db971ca13fbd6bdfb5f2 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0fki-1930000000-f3933f38127537d9d178 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0089-9000000000-0ff95cc18b5212b1c153 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0uk9-8900000000-25af6f5f252b501941e0 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0uxr-1900000000-499115725bc286b22b97 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0uk9-9800000000-2846e6524438703ab54d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0ab9-9000000000-d101f60f5f0933b1cc22 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0a4l-9000000000-b4fce69a41761f7bd63e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0006-9000000000-235022305b68ba272d34 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-106r-9700000000-6ecfd43a641703f25d64 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ab9-9100000000-b709fbaf63ddc2f4c0df | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-76fb37fe98b182da1a5b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-2900000000-3208832c46b361f3099e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-8900000000-2ec3d4f8ca96f4005562 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-9000000000-045583f642e304aeaaf7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00xr-9700000000-fdbd4f0e5bb81c5569f4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9000000000-880db7b265999a2be6b5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-b2689f286fc18087ebce | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-1900000000-de9219963e7858b4c55a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-4900000000-2a4facf9f300cca66157 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9000000000-d4591f1517185fa3d719 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|