| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:22:28 UTC |
|---|
| Update Date | 2020-04-22 15:17:35 UTC |
|---|
| BMDB ID | BMDB0006486 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 5-Methyldihydrofolic acid |
|---|
| Description | 5-Methyldihydrofolic acid, also known as 5-methyl-5,8-dihydrofolate, belongs to the class of organic compounds known as dihydrofolic acids and derivatives. These are heterocyclic compounds based on the 5,6-dihydropteroic acid/7,8-dihydropteroic acid skeleton conjugated with one or more L-glutamic acid units (or derivative thereof). Based on a literature review a significant number of articles have been published on 5-Methyldihydrofolic acid. |
|---|
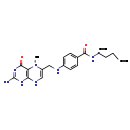
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2S)-2-[(4-{[(2-amino-5-methyl-4-oxo-1,4,5,8-tetrahydropteridin-6-yl)methyl]amino}phenyl)formamido]pentanedioic acid | ChEBI | | 5-Methyl-5,8-dihydrofolic acid | ChEBI | | 5-Methyldihydrofolate | ChEBI | | (2S)-2-[(4-{[(2-amino-5-methyl-4-oxo-1,4,5,8-tetrahydropteridin-6-yl)methyl]amino}phenyl)formamido]pentanedioate | Generator | | 5-Methyl-5,8-dihydrofolate | Generator | | N-[P-[[(2-aminodihydro-4-Hydroxy-5-methyl-6-pteridinyl)methyl]amino]benzoyl]- glutamic acid | HMDB |
|
|---|
| Chemical Formula | C20H23N7O6 |
|---|
| Average Molecular Weight | 457.4399 |
|---|
| Monoisotopic Molecular Weight | 457.170981503 |
|---|
| IUPAC Name | (2S)-2-[(4-{[(2-amino-5-methyl-4-oxo-1,4,5,8-tetrahydropteridin-6-yl)methyl]amino}phenyl)formamido]pentanedioic acid |
|---|
| Traditional Name | (2S)-2-[(4-{[(2-amino-5-methyl-4-oxo-1,8-dihydropteridin-6-yl)methyl]amino}phenyl)formamido]pentanedioic acid |
|---|
| CAS Registry Number | 59904-24-4 |
|---|
| SMILES | CN1C(CNC2=CC=C(C=C2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)=CNC2=C1C(=O)N=C(N)N2 |
|---|
| InChI Identifier | InChI=1S/C20H23N7O6/c1-27-12(9-23-16-15(27)18(31)26-20(21)25-16)8-22-11-4-2-10(3-5-11)17(30)24-13(19(32)33)6-7-14(28)29/h2-5,9,13,22H,6-8H2,1H3,(H,24,30)(H,28,29)(H,32,33)(H4,21,23,25,26,31)/t13-/m0/s1 |
|---|
| InChI Key | VWNDXSYYCICZGD-ZDUSSCGKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dihydrofolic acids and derivatives. These are heterocyclic compounds based on the 5,6-dihydropteroic acid/7,8-dihydropteroic acid skeleton conjugated with one or more L-glutamic acid units (or derivative thereof). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Pterins and derivatives |
|---|
| Direct Parent | Dihydrofolic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dihydrofolic acid or derivatives
- Glutamic acid or derivatives
- Hippuric acid or derivatives
- N-acyl-alpha amino acid or derivatives
- Hippuric acid
- N-acyl-alpha-amino acid
- Alpha-amino acid or derivatives
- Aminobenzamide
- Aminobenzoic acid or derivatives
- Benzamide
- Benzoic acid or derivatives
- Benzoyl
- Tertiary aliphatic/aromatic amine
- Aniline or substituted anilines
- Phenylalkylamine
- Aminopyrimidine
- Pyrimidone
- Secondary aliphatic/aromatic amine
- Dicarboxylic acid or derivatives
- Benzenoid
- Monocyclic benzene moiety
- Pyrimidine
- Vinylogous amide
- Heteroaromatic compound
- Tertiary amine
- Amino acid or derivatives
- Amino acid
- Secondary carboxylic acid amide
- Carboxamide group
- Enamine
- Carboxylic acid
- Carboxylic acid derivative
- Secondary amine
- Azacycle
- Organic nitrogen compound
- Carbonyl group
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cytoplasm
- Lysosome
- Mitochondria
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03di-1444900000-3d359a64f380caa37e40 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-000i-4023390000-d0c5ce85f9ff7bf11058 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0302900000-858fd7ea200bd40564e3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0924300000-3bee881ebce2cd040c1a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-0920000000-988f5acd079eb44b523d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4r-0000900000-facf36af51a3d5028489 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08g0-1245900000-c9a48033185f257663cf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9751300000-6fb9d636c4cfbe8bf60f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0aor-0005900000-35330906be5be391a7f6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kf-2419300000-5adaf44ea9ac5e68290f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udl-4931100000-229e7d53e37b00ca9412 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-08fu-0107900000-a6cec4ccd7a3fa222ac9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-1209100000-6f8d2f3e3438b853b6f5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ox-0913000000-f5d6b93200e0d804f12e | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|