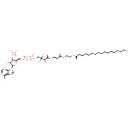| DG(20:0/17:0/0:0) + Heptadecanoyl CoA → TG(20:0/17:0/17:0) + Coenzyme A | details |
| DG(20:0/18:0/0:0) + Heptadecanoyl CoA → TG(20:0/18:0/17:0) + Coenzyme A | details |
| DG(20:0/15:0/0:0) + Heptadecanoyl CoA → TG(20:0/15:0/17:0) + Coenzyme A | details |
| DG(19:0/21:0/0:0) + Heptadecanoyl CoA → TG(19:0/21:0/17:0) + Coenzyme A | details |
| DG(20:0/20:0/0:0) + Heptadecanoyl CoA → TG(20:0/20:0/17:0) + Coenzyme A | details |
| DG(20:0/12:0/0:0) + Heptadecanoyl CoA → TG(20:0/12:0/17:0) + Coenzyme A | details |
| DG(19:0/22:0/0:0) + Heptadecanoyl CoA → TG(19:0/22:0/17:0) + Coenzyme A | details |
| DG(20:0/10:0/0:0) + Heptadecanoyl CoA → TG(20:0/10:0/17:0) + Coenzyme A | details |
| DG(21:0/13:0/0:0) + Heptadecanoyl CoA → TG(21:0/13:0/17:0) + Coenzyme A | details |
| DG(21:0/10:0/0:0) + Heptadecanoyl CoA → TG(21:0/10:0/17:0) + Coenzyme A | details |
| DG(21:0/14:0/0:0) + Heptadecanoyl CoA → TG(21:0/14:0/17:0) + Coenzyme A | details |
| DG(21:0/16:0/0:0) + Heptadecanoyl CoA → TG(21:0/16:0/17:0) + Coenzyme A | details |
| DG(21:0/15:0/0:0) + Heptadecanoyl CoA → TG(21:0/15:0/17:0) + Coenzyme A | details |
| DG(21:0/17:0/0:0) + Heptadecanoyl CoA → TG(21:0/17:0/17:0) + Coenzyme A | details |
| DG(21:0/22:0/0:0) + Heptadecanoyl CoA → TG(21:0/22:0/17:0) + Coenzyme A | details |
| DG(21:0/18:0/0:0) + Heptadecanoyl CoA → TG(21:0/18:0/17:0) + Coenzyme A | details |
| DG(22:0/18:0/0:0) + Heptadecanoyl CoA → TG(22:0/18:0/17:0) + Coenzyme A | details |
| DG(22:0/13:0/0:0) + Heptadecanoyl CoA → TG(22:0/13:0/17:0) + Coenzyme A | details |
| DG(22:0/17:0/0:0) + Heptadecanoyl CoA → TG(22:0/17:0/17:0) + Coenzyme A | details |
| DG(22:0/22:0/0:0) + Heptadecanoyl CoA → TG(22:0/22:0/17:0) + Coenzyme A | details |
| DG(10:0/18:0/0:0) + Heptadecanoyl CoA → TG(10:0/18:0/17:0) + Coenzyme A | details |
| DG(8:0/18:0/0:0) + Heptadecanoyl CoA → TG(8:0/18:0/17:0) + Coenzyme A | details |
| DG(10:0/8:0/0:0) + Heptadecanoyl CoA → TG(10:0/8:0/17:0) + Coenzyme A | details |
| DG(10:0/16:0/0:0) + Heptadecanoyl CoA → TG(10:0/16:0/17:0) + Coenzyme A | details |
| DG(10:0/15:0/0:0) + Heptadecanoyl CoA → TG(10:0/15:0/17:0) + Coenzyme A | details |
| DG(8:0/13:0/0:0) + Heptadecanoyl CoA → TG(8:0/13:0/17:0) + Coenzyme A | details |
| DG(8:0/19:0/0:0) + Heptadecanoyl CoA → TG(8:0/19:0/17:0) + Coenzyme A | details |
| DG(10:0/14:0/0:0) + Heptadecanoyl CoA → TG(10:0/14:0/17:0) + Coenzyme A | details |
| DG(8:0/14:0/0:0) + Heptadecanoyl CoA → TG(8:0/14:0/17:0) + Coenzyme A | details |
| DG(8:0/15:0/0:0) + Heptadecanoyl CoA → TG(8:0/15:0/17:0) + Coenzyme A | details |
| DG(10:0/17:0/0:0) + Heptadecanoyl CoA → TG(10:0/17:0/17:0) + Coenzyme A | details |
| DG(8:0/17:0/0:0) + Heptadecanoyl CoA → TG(8:0/17:0/17:0) + Coenzyme A | details |
| DG(10:0/19:0/0:0) + Heptadecanoyl CoA → TG(10:0/19:0/17:0) + Coenzyme A | details |
| DG(19:0/a-21:0/0:0) + Heptadecanoyl CoA → TG(19:0/a-21:0/17:0) + Coenzyme A | details |
| DG(19:0/a-25:0/0:0) + Heptadecanoyl CoA → TG(19:0/a-25:0/17:0) + Coenzyme A | details |
| DG(19:0/i-21:0/0:0) + Heptadecanoyl CoA → TG(19:0/i-21:0/17:0) + Coenzyme A | details |
| DG(19:0/i-22:0/0:0) + Heptadecanoyl CoA → TG(19:0/i-22:0/17:0) + Coenzyme A | details |
| DG(19:0/i-24:0/0:0) + Heptadecanoyl CoA → TG(19:0/i-24:0/17:0) + Coenzyme A | details |
| DG(a-21:0/12:0/0:0) + Heptadecanoyl CoA → TG(a-21:0/12:0/17:0)[rac] + Coenzyme A | details |
| DG(a-21:0/13:0/0:0) + Heptadecanoyl CoA → TG(a-21:0/13:0/17:0)[rac] + Coenzyme A | details |
| DG(a-21:0/14:0/0:0) + Heptadecanoyl CoA → TG(a-21:0/14:0/17:0)[rac] + Coenzyme A | details |
| DG(a-21:0/15:0/0:0) + Heptadecanoyl CoA → TG(a-21:0/15:0/17:0)[rac] + Coenzyme A | details |
| DG(a-21:0/16:0/0:0) + Heptadecanoyl CoA → TG(a-21:0/16:0/17:0)[rac] + Coenzyme A | details |
| DG(a-21:0/17:0/0:0) + Heptadecanoyl CoA → TG(a-21:0/17:0/17:0)[rac] + Coenzyme A | details |
| DG(a-21:0/a-21:0/0:0) + Heptadecanoyl CoA → TG(a-21:0/a-21:0/17:0)[rac] + Coenzyme A | details |
| DG(a-25:0/12:0/0:0) + Heptadecanoyl CoA → TG(a-25:0/12:0/17:0)[rac] + Coenzyme A | details |
| DG(a-25:0/13:0/0:0) + Heptadecanoyl CoA → TG(a-25:0/13:0/17:0)[rac] + Coenzyme A | details |
| DG(a-25:0/14:0/0:0) + Heptadecanoyl CoA → TG(a-25:0/14:0/17:0)[rac] + Coenzyme A | details |
| DG(a-25:0/15:0/0:0) + Heptadecanoyl CoA → TG(a-25:0/15:0/17:0)[rac] + Coenzyme A | details |
| DG(a-25:0/16:0/0:0) + Heptadecanoyl CoA → TG(a-25:0/16:0/17:0)[rac] + Coenzyme A | details |
| DG(a-25:0/17:0/0:0) + Heptadecanoyl CoA → TG(a-25:0/17:0/17:0)[rac] + Coenzyme A | details |
| DG(a-25:0/a-25:0/0:0) + Heptadecanoyl CoA → TG(a-25:0/a-25:0/17:0)[rac] + Coenzyme A | details |
| DG(i-19:0/i-21:0/0:0) + Heptadecanoyl CoA → TG(i-19:0/i-21:0/17:0) + Coenzyme A | details |
| DG(i-19:0/i-22:0/0:0) + Heptadecanoyl CoA → TG(i-19:0/i-22:0/17:0) + Coenzyme A | details |
| DG(i-19:0/i-24:0/0:0) + Heptadecanoyl CoA → TG(i-19:0/i-24:0/17:0) + Coenzyme A | details |
| DG(i-20:0/12:0/0:0) + Heptadecanoyl CoA → TG(i-20:0/12:0/17:0) + Coenzyme A | details |
| DG(i-20:0/13:0/0:0) + Heptadecanoyl CoA → TG(i-20:0/13:0/17:0) + Coenzyme A | details |
| DG(i-20:0/14:0/0:0) + Heptadecanoyl CoA → TG(i-20:0/14:0/17:0) + Coenzyme A | details |
| DG(i-20:0/15:0/0:0) + Heptadecanoyl CoA → TG(i-20:0/15:0/17:0) + Coenzyme A | details |
| DG(i-20:0/16:0/0:0) + Heptadecanoyl CoA → TG(i-20:0/16:0/17:0) + Coenzyme A | details |
| DG(i-20:0/17:0/0:0) + Heptadecanoyl CoA → TG(i-20:0/17:0/17:0) + Coenzyme A | details |
| DG(i-20:0/i-20:0/0:0) + Heptadecanoyl CoA → TG(i-20:0/i-20:0/17:0) + Coenzyme A | details |
| DG(i-21:0/12:0/0:0) + Heptadecanoyl CoA → TG(i-21:0/12:0/17:0) + Coenzyme A | details |
| DG(i-21:0/13:0/0:0) + Heptadecanoyl CoA → TG(i-21:0/13:0/17:0) + Coenzyme A | details |
| DG(i-21:0/14:0/0:0) + Heptadecanoyl CoA → TG(i-21:0/14:0/17:0) + Coenzyme A | details |
| DG(i-21:0/15:0/0:0) + Heptadecanoyl CoA → TG(i-21:0/15:0/17:0) + Coenzyme A | details |
| DG(i-21:0/16:0/0:0) + Heptadecanoyl CoA → TG(i-21:0/16:0/17:0) + Coenzyme A | details |
| DG(i-21:0/17:0/0:0) + Heptadecanoyl CoA → TG(i-21:0/17:0/17:0) + Coenzyme A | details |
| DG(i-21:0/i-21:0/0:0) + Heptadecanoyl CoA → TG(i-21:0/i-21:0/17:0) + Coenzyme A | details |
| DG(i-22:0/12:0/0:0) + Heptadecanoyl CoA → TG(i-22:0/12:0/17:0) + Coenzyme A | details |
| DG(i-22:0/13:0/0:0) + Heptadecanoyl CoA → TG(i-22:0/13:0/17:0) + Coenzyme A | details |
| DG(i-22:0/14:0/0:0) + Heptadecanoyl CoA → TG(i-22:0/14:0/17:0) + Coenzyme A | details |
| DG(i-22:0/15:0/0:0) + Heptadecanoyl CoA → TG(i-22:0/15:0/17:0) + Coenzyme A | details |
| DG(i-22:0/16:0/0:0) + Heptadecanoyl CoA → TG(i-22:0/16:0/17:0) + Coenzyme A | details |
| DG(i-22:0/17:0/0:0) + Heptadecanoyl CoA → TG(i-22:0/17:0/17:0) + Coenzyme A | details |
| DG(i-22:0/i-22:0/0:0) + Heptadecanoyl CoA → TG(i-22:0/i-22:0/17:0) + Coenzyme A | details |
| DG(i-24:0/12:0/0:0) + Heptadecanoyl CoA → TG(i-24:0/12:0/17:0) + Coenzyme A | details |
| DG(i-24:0/13:0/0:0) + Heptadecanoyl CoA → TG(i-24:0/13:0/17:0) + Coenzyme A | details |
| DG(i-24:0/14:0/0:0) + Heptadecanoyl CoA → TG(i-24:0/14:0/17:0) + Coenzyme A | details |
| DG(i-24:0/15:0/0:0) + Heptadecanoyl CoA → TG(i-24:0/15:0/17:0) + Coenzyme A | details |
| DG(i-24:0/16:0/0:0) + Heptadecanoyl CoA → TG(i-24:0/16:0/17:0) + Coenzyme A | details |
| DG(i-24:0/17:0/0:0) + Heptadecanoyl CoA → TG(i-24:0/17:0/17:0) + Coenzyme A | details |
| DG(i-24:0/i-24:0/0:0) + Heptadecanoyl CoA → TG(i-24:0/i-24:0/17:0) + Coenzyme A | details |