| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:23:34 UTC |
|---|
| Update Date | 2020-04-22 15:17:55 UTC |
|---|
| BMDB ID | BMDB0006577 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Lacto-N-fucopentaose-2 |
|---|
| Description | Lacto-N-fucopentaose-2, also known as LNFP II, belongs to the class of organic compounds known as oligosaccharides. These are carbohydrates made up of 3 to 10 monosaccharide units linked to each other through glycosidic bonds. Based on a literature review very few articles have been published on Lacto-N-fucopentaose-2. |
|---|
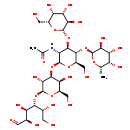
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| LNFP II | MeSH | | Lacto-N-fucopentaose II | MeSH | | Lea penta | HMDB | | LNF II | HMDB | | O-6-Deoxy-alpha-L-galactopyranosyl-(1->4)-O-[beta-D-galactopyranosyl-(1->3)]-O-2-(acetylamino)-2-deoxy-b-D-glucopyranosyl-(1->3)-O-b-D-galactopyranosyl-(1->4)- D-glucose | HMDB | | O-6-Deoxy-alpha-L-galactopyranosyl-(1->4)-O-[beta-D-galactopyranosyl-(1->3)]-O-2-(acetylamino)-2-deoxy-beta-D-glucopyranosyl-(1->3)-O-beta-D-galactopyranosyl-(1->4)- D-glucose | HMDB | | O-6-Deoxy-alpha-L-galactopyranosyl-(1->4)-O-[beta-D-galactopyranosyl-(1->3)]-O-2-acetamido-2-deoxy-beta-D-glucopyranosyl-(1->3)-O-b-D-galactopyranosyl-(1->4)-D-glucopyranose | HMDB | | O-6-Deoxy-alpha-L-galactopyranosyl-(1->4)-O-[beta-D-galactopyranosyl-(1->3)]-O-2-acetamido-2-deoxy-beta-D-glucopyranosyl-(1->3)-O-beta-D-galactopyranosyl-(1->4)-D-glucopyranose | HMDB | | O-6-Deoxy-alpha-L-galactopyranosyl-(1->4)-O-[beta-delta-galactopyranosyl-(1->3)]-O-2-(acetylamino)-2-deoxy-beta-delta-glucopyranosyl-(1->3)-O-beta-delta-galactopyranosyl-(1->4)- D-glucose | HMDB | | O-6-Deoxy-alpha-L-galactopyranosyl-(1->4)-O-[beta-delta-galactopyranosyl-(1->3)]-O-2-acetamido-2-deoxy-beta-delta-glucopyranosyl-(1->3)-O-beta-delta-galactopyranosyl-(1->4)-delta-glucopyranose | HMDB | | N-[(2S,3R,4R,5S,6R)-2-{[(2R,3S,4S,5R,6S)-3,5-dihydroxy-2-(hydroxymethyl)-6-{[(2R,3R,4R,5R)-1,2,4,5-tetrahydroxy-6-oxohexan-3-yl]oxy}oxan-4-yl]oxy}-6-(hydroxymethyl)-4-{[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-5-{[(2S,3S,4R,5S,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}oxan-3-yl]ethanimidate | Generator, HMDB |
|
|---|
| Chemical Formula | C32H55NO25 |
|---|
| Average Molecular Weight | 853.7708 |
|---|
| Monoisotopic Molecular Weight | 853.306316315 |
|---|
| IUPAC Name | N-[(2S,3R,4R,5S,6R)-2-{[(2R,3S,4S,5R,6S)-3,5-dihydroxy-2-(hydroxymethyl)-6-{[(2R,3R,4R,5R)-1,2,4,5-tetrahydroxy-6-oxohexan-3-yl]oxy}oxan-4-yl]oxy}-6-(hydroxymethyl)-4-{[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-5-{[(2S,3S,4R,5S,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}oxan-3-yl]acetamide |
|---|
| Traditional Name | N-[(2S,3R,4R,5S,6R)-2-{[(2R,3S,4S,5R,6S)-3,5-dihydroxy-2-(hydroxymethyl)-6-{[(2R,3R,4R,5R)-1,2,4,5-tetrahydroxy-6-oxohexan-3-yl]oxy}oxan-4-yl]oxy}-6-(hydroxymethyl)-4-{[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-5-{[(2S,3S,4R,5S,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}oxan-3-yl]acetamide |
|---|
| CAS Registry Number | 21973-23-9 |
|---|
| SMILES | C[C@@H]1O[C@@H](O[C@@H]2[C@@H](CO)O[C@@H](O[C@H]3[C@@H](O)[C@@H](CO)O[C@@H](O[C@H]([C@H](O)CO)[C@H](O)[C@@H](O)C=O)[C@@H]3O)[C@H](NC(C)=O)[C@H]2O[C@@H]2O[C@H](CO)[C@H](O)[C@H](O)[C@H]2O)[C@@H](O)[C@H](O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C32H55NO25/c1-8-16(42)20(46)22(48)30(51-8)56-26-14(7-38)54-29(15(33-9(2)39)27(26)57-31-23(49)21(47)18(44)12(5-36)52-31)58-28-19(45)13(6-37)53-32(24(28)50)55-25(11(41)4-35)17(43)10(40)3-34/h3,8,10-32,35-38,40-50H,4-7H2,1-2H3,(H,33,39)/t8-,10-,11+,12+,13+,14+,15+,16+,17+,18-,19-,20+,21-,22-,23+,24+,25+,26+,27+,28-,29-,30-,31-,32-/m0/s1 |
|---|
| InChI Key | FKADDOYBRRMBPP-QKPOUJQKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligosaccharides. These are carbohydrates made up of 3 to 10 monosaccharide units linked to each other through glycosidic bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Oligosaccharides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oligosaccharide
- Fatty acyl glycoside
- N-acyl-alpha-hexosamine
- Alkyl glycoside
- Glycosyl compound
- O-glycosyl compound
- Beta-hydroxy aldehyde
- Fatty acyl
- Oxane
- Acetamide
- Alpha-hydroxyaldehyde
- Carboxamide group
- Secondary carboxylic acid amide
- Secondary alcohol
- Acetal
- Carboxylic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Hydrocarbon derivative
- Alcohol
- Organic oxide
- Carbonyl group
- Organopnictogen compound
- Aldehyde
- Primary alcohol
- Organic nitrogen compound
- Organonitrogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-007x-0701019150-61c29c3675d7faea183c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01po-3902047100-ba36751bb3cacf446ed5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01po-5900077210-ab7eefa27af17fee3901 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-9433005780-8c12cd7bbf22c035e132 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05e9-8806029180-3b4b2e609369de380435 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01t9-6933200000-c502b58ab4cb19add026 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0200049130-147a7f4703369dc2c2e1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0007-6600089250-fc465433cebf68ea53ef | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ow-9600030100-d7de39285de6561e05ba | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-3400000690-f3d4e9205ccc4d735a3e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0m07-5600000940-5ed742b08f1ab1a76990 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000022000-f99b9d21cbe7efbc0580 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|