| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:24:53 UTC |
|---|
| Update Date | 2020-05-11 20:41:32 UTC |
|---|
| BMDB ID | BMDB0006695 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Prolylhydroxyproline |
|---|
| Description | Prolylhydroxyproline, also known as L-pro-L-hyp, belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. Based on a literature review a significant number of articles have been published on Prolylhydroxyproline. |
|---|
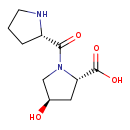
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| L-Pro-L-hyp | ChEBI | | L-Prolyl-L-hydroxyproline | ChEBI | | (4R)-L-Prolyl-4-hydroxy-L-proline | HMDB | | 4-Hydroxy-1-L-prolyl-proline | HMDB | | L-4-Hydroxy-1-L-prolyl-proline | HMDB | | Proline-hydroxyproline | HMDB | | trans-4-Hydroxy-1-L-prolyl-proline | HMDB | | Prolylhydroxyproline | ChEBI | | Pro-hydroxy-Pro | HMDB | | Pro-Hyp | HMDB | | Proline hydroxyproline dipeptide | HMDB | | Proline-hydroxyproline dipeptide | HMDB |
|
|---|
| Chemical Formula | C10H16N2O4 |
|---|
| Average Molecular Weight | 228.245 |
|---|
| Monoisotopic Molecular Weight | 228.11100701 |
|---|
| IUPAC Name | (2S,4R)-4-hydroxy-1-[(2S)-pyrrolidine-2-carbonyl]pyrrolidine-2-carboxylic acid |
|---|
| Traditional Name | proline-hydroxyproline |
|---|
| CAS Registry Number | 18684-24-7 |
|---|
| SMILES | O[C@@H]1C[C@H](N(C1)C(=O)[C@@H]1CCCN1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C10H16N2O4/c13-6-4-8(10(15)16)12(5-6)9(14)7-2-1-3-11-7/h6-8,11,13H,1-5H2,(H,15,16)/t6-,7+,8+/m1/s1 |
|---|
| InChI Key | ONPXCLZMBSJLSP-CSMHCCOUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Dipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-dipeptide
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Proline or derivatives
- N-acyl-l-alpha-amino acid
- Alpha-amino acid amide
- Alpha-amino acid or derivatives
- N-acylpyrrolidine
- Pyrrolidine carboxylic acid
- Pyrrolidine carboxylic acid or derivatives
- Pyrrolidine-2-carboxamide
- Tertiary carboxylic acid amide
- Pyrrolidine
- Secondary alcohol
- Amino acid
- Amino acid or derivatives
- Carboxamide group
- Secondary amine
- Azacycle
- Organoheterocyclic compound
- Carboxylic acid
- Secondary aliphatic amine
- Monocarboxylic acid or derivatives
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxide
- Alcohol
- Organonitrogen compound
- Carbonyl group
- Organooxygen compound
- Amine
- Organic nitrogen compound
- Organic oxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00dj-9200000000-cefb6332c4f93d2283b3 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0a4l-2692000000-7b1c4c7371558705bfb0 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-00di-9200000000-6af4dc9ae133d399503c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-0b90-2900000000-84bcb537803c3e383c98 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-1490000000-d607eb5709327adc37c3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0229-9520000000-53efc51e1a53a9bb992e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9200000000-c54979309cb483b47143 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0059-0690000000-385af9502303330863e3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05o0-3920000000-648587bfd6a99e5a6a25 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0239-9200000000-e801efd4fdf93e14e8f2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01u1-4890000000-317a4ceb981d4c1d3a2c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-2900000000-cb814d27ee8198848547 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-df69d58c4ec35c77f011 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01t9-5690000000-afc10370af26972ee32d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0229-9610000000-4039cba60bbe790e2e1c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9300000000-0dd3ad4a047284fe2d79 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|