| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:24:58 UTC |
|---|
| Update Date | 2020-04-22 15:18:16 UTC |
|---|
| BMDB ID | BMDB0006700 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 3-O-fucopyranosyl-2-acetamido-2-deoxyglucopyranose |
|---|
| Description | 3-O-fucopyranosyl-2-acetamido-2-deoxyglucopyranose belongs to the class of organic compounds known as fatty acyl glycosides of mono- and disaccharides. Fatty acyl glycosides of mono- and disaccharides are compounds composed of a mono- or disaccharide moiety linked to one hydroxyl group of a fatty alcohol or of a phosphorylated alcohol (phosphoprenols), a hydroxy fatty acid or to one carboxyl group of a fatty acid (ester linkage) or to an amino alcohol. Based on a literature review a small amount of articles have been published on 3-O-fucopyranosyl-2-acetamido-2-deoxyglucopyranose. |
|---|
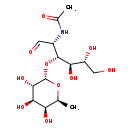
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| -O-Fucopyranosyl-2-acetamido-2-deoxyglucopyranose | HMDB | | 2-(acetylamino)-2-Deoxy-3-O-(6-deoxy-alpha-L-galactopyranosyl)- D-glucose | HMDB | | 2-acetamido-2-Deoxy-3-O-alpha-L-fucopyranosyl-D-glucose | HMDB | | 2-acetamido-2-Deoxy-3-O-alpha-L-fucopyranosyl-delta-glucose | HMDB | | Fuc-1-3-glcnac | MeSH, HMDB | | N-[(2R,3R,4R,5R)-4,5,6-Trihydroxy-1-oxo-3-{[(2S,3S,4R,5S,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}hexan-2-yl]ethanimidate | Generator, HMDB | | 3-O-Fucopyranosyl-2-acetamido-2-deoxyglucopyranose | MeSH |
|
|---|
| Chemical Formula | C14H25NO10 |
|---|
| Average Molecular Weight | 367.349 |
|---|
| Monoisotopic Molecular Weight | 367.147846025 |
|---|
| IUPAC Name | N-[(2R,3R,4R,5R)-4,5,6-trihydroxy-1-oxo-3-{[(2S,3S,4R,5S,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}hexan-2-yl]acetamide |
|---|
| Traditional Name | N-[(2R,3R,4R,5R)-4,5,6-trihydroxy-1-oxo-3-{[(2S,3S,4R,5S,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}hexan-2-yl]acetamide |
|---|
| CAS Registry Number | 52630-68-9 |
|---|
| SMILES | C[C@@H]1O[C@@H](O[C@@H]([C@H](O)[C@H](O)CO)[C@@H](NC(C)=O)C=O)[C@@H](O)[C@H](O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C14H25NO10/c1-5-9(20)11(22)12(23)14(24-5)25-13(10(21)8(19)4-17)7(3-16)15-6(2)18/h3,5,7-14,17,19-23H,4H2,1-2H3,(H,15,18)/t5-,7-,8+,9+,10+,11+,12-,13+,14-/m0/s1 |
|---|
| InChI Key | YDWJUIXDZSJZHH-MYCHVAHWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as fatty acyl glycosides of mono- and disaccharides. Fatty acyl glycosides of mono- and disaccharides are compounds composed of a mono- or disaccharide moiety linked to one hydroxyl group of a fatty alcohol or of a phosphorylated alcohol (phosphoprenols), a hydroxy fatty acid or to one carboxyl group of a fatty acid (ester linkage) or to an amino alcohol. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acyl glycosides |
|---|
| Direct Parent | Fatty acyl glycosides of mono- and disaccharides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fatty acyl glycoside of mono- or disaccharide
- Alkyl glycoside
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Amino saccharide
- Monosaccharide
- Oxane
- Acetamide
- Carboxamide group
- Secondary carboxylic acid amide
- Secondary alcohol
- Acetal
- Carboxylic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Aldehyde
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Alcohol
- Organic oxygen compound
- Carbonyl group
- Organonitrogen compound
- Organooxygen compound
- Primary alcohol
- Organic nitrogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0r2a-9117000000-152a02d05dca63a00a1c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0006-4810239000-ce72b24b328b9b499e97 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uk9-1279000000-52455de188736dc45c79 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-7950000000-02863fcb403f7c83e955 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ik9-9530000000-634127d9fc77968aae3d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-11or-4279000000-b0db61113513601ec2ec | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0r01-9663000000-536c8f195acc12187c2e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0bt9-9520000000-238dd2888391a2e9515d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0aor-3469000000-50739234257caab68597 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4j-9724000000-3ab197a084258b0c5492 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9800000000-3c5ba817d27d23e1c608 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0g4i-0967000000-f3759a0bd96671f31c56 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0230-4911000000-374097bd0a76701f5c81 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0759-9850000000-1e7dc0afd1c536bec89f | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|