| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:26:07 UTC |
|---|
| Update Date | 2020-04-22 15:18:37 UTC |
|---|
| BMDB ID | BMDB0006798 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 1,4-b-D-Mannan |
|---|
| Description | 1,4-b-D-Mannan belongs to the class of organic compounds known as oligosaccharides. These are carbohydrates made up of 3 to 10 monosaccharide units linked to each other through glycosidic bonds. Based on a literature review very few articles have been published on 1,4-b-D-Mannan. |
|---|
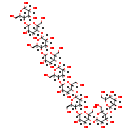
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,4-beta-D-Mannan | HMDB | | 1,4-beta-delta-Mannan | HMDB |
|
|---|
| Chemical Formula | C60H102O51 |
|---|
| Average Molecular Weight | 1639.4213 |
|---|
| Monoisotopic Molecular Weight | 1638.538798986 |
|---|
| IUPAC Name | (3S,4R,5S,6R)-5-{[(3S,4R,5S,6R)-5-{[(3S,4R,5S,6R)-5-{[(3S,4R,5S,6R)-5-{[(3S,4R,5S,6R)-5-{[(3S,4R,5S,6R)-5-{[(3S,4R,5S,6R)-5-{[(3S,4R,5S,6R)-5-{[(3S,4R,5S,6R)-3,4-dihydroxy-6-(hydroxymethyl)-5-{[(3S,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-6-(hydroxymethyl)oxane-2,3,4-triol |
|---|
| Traditional Name | (3S,4R,5S,6R)-5-{[(3S,4R,5S,6R)-5-{[(3S,4R,5S,6R)-5-{[(3S,4R,5S,6R)-5-{[(3S,4R,5S,6R)-5-{[(3S,4R,5S,6R)-5-{[(3S,4R,5S,6R)-5-{[(3S,4R,5S,6R)-5-{[(3S,4R,5S,6R)-3,4-dihydroxy-6-(hydroxymethyl)-5-{[(3S,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-6-(hydroxymethyl)oxane-2,3,4-triol |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | OC[C@H]1OC(O[C@H]2[C@H](O)[C@H](O)C(O[C@H]3[C@H](O)[C@H](O)C(O[C@H]4[C@H](O)[C@H](O)C(O[C@H]5[C@H](O)[C@H](O)C(O[C@H]6[C@H](O)[C@H](O)C(O[C@H]7[C@H](O)[C@H](O)C(O[C@H]8[C@H](O)[C@H](O)C(O[C@H]9[C@H](O)[C@H](O)C(O[C@H]%10[C@H](O)[C@H](O)C(O)O[C@@H]%10CO)O[C@@H]9CO)O[C@@H]8CO)O[C@@H]7CO)O[C@@H]6CO)O[C@@H]5CO)O[C@@H]4CO)O[C@@H]3CO)O[C@@H]2CO)[C@@H](O)[C@@H](O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C60H102O51/c61-1-11-21(71)22(72)33(83)52(94-11)104-43-13(3-63)96-54(35(85)24(43)74)106-45-15(5-65)98-56(37(87)26(45)76)108-47-17(7-67)100-58(39(89)28(47)78)110-49-19(9-69)102-60(41(91)30(49)80)111-50-20(10-70)101-59(40(90)31(50)81)109-48-18(8-68)99-57(38(88)29(48)79)107-46-16(6-66)97-55(36(86)27(46)77)105-44-14(4-64)95-53(34(84)25(44)75)103-42-12(2-62)93-51(92)32(82)23(42)73/h11-92H,1-10H2/t11-,12-,13-,14-,15-,16-,17-,18-,19-,20-,21-,22+,23-,24-,25-,26-,27-,28-,29-,30-,31-,32+,33+,34+,35+,36+,37+,38+,39+,40+,41+,42-,43-,44-,45-,46-,47-,48-,49-,50-,51?,52?,53?,54?,55?,56?,57?,58?,59?,60?/m1/s1 |
|---|
| InChI Key | RJQKKZNUWRIHCS-YDPWGIMBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligosaccharides. These are carbohydrates made up of 3 to 10 monosaccharide units linked to each other through glycosidic bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Oligosaccharides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oligosaccharide
- O-glycosyl compound
- Glycosyl compound
- Oxane
- Secondary alcohol
- Hemiacetal
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Acetal
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0311109011-944406bcab5a3f14fee2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05fr-0422229033-801fd07ee2adc667d8f0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05gl-0913227022-31e9b9fb6512817b44b4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0212139020-04ad00f142ff334599f7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0402129021-39cc47a26cfd6c3433b5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fvi-0915056031-013f53c133a3373898a9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00kr-0000069000-0344d8fe2da1d8f0aa85 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05p9-5212259000-c81f4e330a75b89a9425 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pdr-9516780132-ba7e0b4f273dc0edba4b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-007a-0301209011-9628fc7ded4282fae10b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-007a-0412339011-2f1040d4fabaf84cd432 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000b-7911234001-dda3db40897c4a58d175 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|