| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:26:56 UTC |
|---|
| Update Date | 2020-05-21 16:28:44 UTC |
|---|
| BMDB ID | BMDB0006866 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
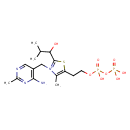
| Common Name | 2-Methyl-1-hydroxypropyl-ThPP |
|---|
| Description | 2-Methyl-1-hydroxypropyl-ThPP belongs to the class of organic compounds known as thiamine phosphates. These are thiamine derivatives in which the hydroxyl group of the ethanol moiety is substituted by a phosphate group. 2-Methyl-1-hydroxypropyl-ThPP is a very strong basic compound (based on its pKa). 2-Methyl-1-hydroxypropyl-ThPP exists in all living organisms, ranging from bacteria to humans. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Hydroxy-2-methylpropyl-thiamine diphosphate | ChEBI | | 1-Hydroxy-2-methylpropyl-thiamine pyrophosphate | ChEBI | | 2-Methyl-1-hydroxypropyl-thiamine diphosphate | ChEBI | | 2-Methyl-1-hydroxypropyl-thiamine pyrophosphate | ChEBI | | 2-Methyl-1-hydroxypropyl-TPP | ChEBI | | 3-[(4-Amino-2-methylpyrimidin-5-yl)methyl]-5-(2-diphosphoethyl)-2-(1-hydroxy-2-methylpropyl)-4-methyl-1,3-thiazol-3-ium | ChEBI | | 1-Hydroxy-2-methylpropyl-thiamine diphosphoric acid | Generator | | 1-Hydroxy-2-methylpropyl-thiamine pyrophosphoric acid | Generator | | 2-Methyl-1-hydroxypropyl-thiamine diphosphoric acid | Generator | | 2-Methyl-1-hydroxypropyl-thiamine pyrophosphoric acid | Generator | | 2-Methyl-1-hydroxypropyl-THPP | ChEBI |
|
|---|
| Chemical Formula | C16H27N4O8P2S |
|---|
| Average Molecular Weight | 497.42 |
|---|
| Monoisotopic Molecular Weight | 497.102482574 |
|---|
| IUPAC Name | 3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-5-(2-{[hydroxy(phosphonooxy)phosphoryl]oxy}ethyl)-2-(1-hydroxy-2-methylpropyl)-4-methyl-1,3-thiazol-3-ium |
|---|
| Traditional Name | 3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-5-(2-{[hydroxy(phosphonooxy)phosphoryl]oxy}ethyl)-2-(1-hydroxy-2-methylpropyl)-4-methyl-1,3-thiazol-3-ium |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CC(C)C(O)C1=[N+](CC2=C(N)N=C(C)N=C2)C(C)=C(CCOP(O)(=O)OP(O)(O)=O)S1 |
|---|
| InChI Identifier | InChI=1S/C16H26N4O8P2S/c1-9(2)14(21)16-20(8-12-7-18-11(4)19-15(12)17)10(3)13(31-16)5-6-27-30(25,26)28-29(22,23)24/h7,9,14,21H,5-6,8H2,1-4H3,(H4-,17,18,19,22,23,24,25,26)/p+1 |
|---|
| InChI Key | SSYCSHKTIOHFEZ-UHFFFAOYSA-O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thiamine phosphates. These are thiamine derivatives in which the hydroxyl group of the ethanol moiety is substituted by a phosphate group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Thiamine phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Thiamine-phosphate
- Organic pyrophosphate
- 2,4,5-trisubstituted 1,3-thiazole
- Aminopyrimidine
- Monoalkyl phosphate
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Imidolactam
- Heteroaromatic compound
- Azole
- Thiazole
- Secondary alcohol
- Azacycle
- Hydrocarbon derivative
- Amine
- Organic oxide
- Primary amine
- Alcohol
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic nitrogen compound
- Aromatic alcohol
- Organic oxygen compound
- Organic cation
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-9813800000-be6335483cae172e16a9 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-004i-7911440000-4b658ddfc94358dd1ffa | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00xs-1368900000-1e4eff19f9ce5ce0973d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00xs-4419100000-89ecb98fc9c3156fe1c2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-3941000000-528910db0245fc831976 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0101900000-a6bad091c8649111d49b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00r2-1207900000-4c68794467db876e9d52 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-2933000000-f325b11cce71f2cc69ce | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|