| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:27:32 UTC |
|---|
| Update Date | 2020-04-22 15:19:03 UTC |
|---|
| BMDB ID | BMDB0006904 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
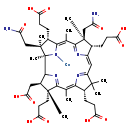
| Common Name | Cob(I)yrinate a,c diamide |
|---|
| Description | Cob(I)yrinate a,c diamide, also known as cob(i)yrinic acid a,c-diamide, belongs to the class of organic compounds known as precorrins. These are intermediates formed by methylation at one or more of the four rings prior to the formation of the macrocyclic corrin ring. Based on a literature review very few articles have been published on Cob(I)yrinate a,c diamide. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Cob(I)yrinate diamide | Kegg | | Cob(I)yrinic acid a,c-diamide | Kegg | | Cob(I)yrinic acid diamide | Generator | | Cob(I)yrinate a,c-diamide | Generator | | Cob(I)yrinic acid a,c diamide | Generator |
|
|---|
| Chemical Formula | C45H61CoN6O12 |
|---|
| Average Molecular Weight | 936.932 |
|---|
| Monoisotopic Molecular Weight | 936.36794664 |
|---|
| IUPAC Name | [(1R,3R,4R,8S,13S,14S,18S,19S)-14,19-bis(carbamoylmethyl)-4,8,13,18-tetrakis(2-carboxyethyl)-3-(carboxymethyl)-1,4,6,9,9,14,16,19-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1²,⁵.1⁷,¹⁰.1¹²,¹⁵]tricosa-5(23),6,10(22),11,15(21),16-hexaen-20-yl]cobalt |
|---|
| Traditional Name | [(1R,3R,4R,8S,13S,14S,18S,19S)-14,19-bis(carbamoylmethyl)-4,8,13,18-tetrakis(2-carboxyethyl)-3-(carboxymethyl)-1,4,6,9,9,14,16,19-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1²,⁵.1⁷,¹⁰.1¹²,¹⁵]tricosa-5(23),6,10(22),11,15(21),16-hexaen-20-yl]cobalt |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [Co]N1\C2=C(C)/C3=N/C(=C\C4=N\C(=C(C)/C5=NC([C@H](CC(O)=O)[C@@]5(C)CCC(O)=O)[C@@]1(C)[C@@](C)(CC(N)=O)[C@@H]2CCC(O)=O)\[C@@H](CCC(O)=O)C4(C)C)/[C@@H](CCC(O)=O)[C@]3(C)CC(N)=O |
|---|
| InChI Identifier | InChI=1S/C45H62N6O12.Co/c1-21-36-24(10-13-32(56)57)41(3,4)28(49-36)18-27-23(9-12-31(54)55)43(6,19-29(46)52)39(48-27)22(2)37-25(11-14-33(58)59)44(7,20-30(47)53)45(8,51-37)40-26(17-35(62)63)42(5,38(21)50-40)16-15-34(60)61;/h18,23-26,40H,9-17,19-20H2,1-8H3,(H10,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63);/q;+1/p-1/t23-,24-,25-,26+,40?,42-,43+,44+,45+;/m1./s1 |
|---|
| InChI Key | NKLHEMWEQJCPPF-YYYLUSCNSA-M |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as precorrins. These are intermediates formed by methylation at one or more of the four rings prior to the formation of the macrocyclic corrin ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Corrinoids |
|---|
| Direct Parent | Precorrins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Precorrin
- Metallotetrapyrrole skeleton
- Pentacarboxylic acid or derivatives
- Fatty amide
- Fatty acyl
- Pyrrolidine
- Pyrroline
- Carboxamide group
- Ketimine
- Primary carboxylic acid amide
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Azacycle
- Organic transition metal salt
- Organic metal salt
- Carboxylic acid derivative
- Carboxylic acid
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Carbonyl group
- Imine
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Organic salt
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00mo-0000000094-3d6e4ce7f8817f98cd4f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00tf-0000000092-38588470e15aa828745c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f7k-0000000090-c323c3865a18a80f498b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000f-0000000096-722a9c0c2d8af90c6ce7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dj-0000000091-496e6ce7a3b7dd183ef4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006y-4000000090-fe5170e3906f83771081 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000000009-40a9ddf2a8a42c740943 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00ku-1000000193-a58d27de536d92012d5d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014j-0000000191-4a95123c692454052509 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0hg9-0000000039-719e3f3aa129c1bd48c0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0wmi-0000000093-e9b89c379897d4d94bcb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aor-5000000190-d4444dc338b35e0bd39a | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|