| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:45:53 UTC |
|---|
| Update Date | 2020-05-21 16:27:38 UTC |
|---|
| BMDB ID | BMDB0007860 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
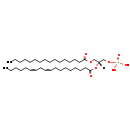
| Common Name | PA(16:0/18:2(9Z,12Z)) |
|---|
| Description | PA(16:0/18:2(9Z,12Z)), also known as Pa(16:0/18:2(9z,12z)) or PA(16:0/18:2), belongs to the class of organic compounds known as 1,2-diacylglycerol-3-phosphates. These are glycerol-3-phosphates in which the glycerol moiety is bonded to two aliphatic chains through ester linkages. Thus, PA(16:0/18:2(9Z,12Z)) is considered to be a glycerophosphate lipid molecule. PA(16:0/18:2(9Z,12Z)) is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. PA(16:0/18:2(9Z,12Z)) exists in all living organisms, ranging from bacteria to humans. PA(16:0/18:2(9Z,12Z)) participates in a number of enzymatic reactions, within cattle. In particular, PA(16:0/18:2(9Z,12Z)) can be biosynthesized from lpa(16:0/0:0) and linoleoyl-CoA through its interaction with the enzyme 1-acyl-sn-glycerol-3-phosphate acyltransferase epsilon. In addition, Cytidine triphosphate and PA(16:0/18:2(9Z,12Z)) can be converted into CDP-DG(16:0/18:2(9Z,12Z)) through its interaction with the enzyme phosphatidate cytidylyltransferase 2. In cattle, PA(16:0/18:2(9Z,12Z)) is involved in the metabolic pathway called cardiolipin biosynthesis CL(16:0/18:2(9Z,12Z)/16:0/18:2(9Z,12Z)) pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-16:0-2-18:2-Phosphatidic acid | ChEBI | | 1-Palmitoyl-2-linoleoyl-sn-glycero-3-phosphate | ChEBI | | 16:0-18:2-PA | ChEBI | | 2-Linoleoyl-1-palmitoyl-sn-glycero-3-phosphate | ChEBI | | PA(16:0/18:2) | ChEBI | | PA(16:0/18:2OMEGA6) | ChEBI | | PA(34:2) | ChEBI | | Phosphatidic acid(16:0/18:2) | ChEBI | | Phosphatidic acid(16:0/18:2omega6) | ChEBI | | Phosphatidic acid(34:2) | ChEBI | | 1-16:0-2-18:2-Phosphatidate | Generator | | 1-Palmitoyl-2-linoleoyl-sn-glycero-3-phosphoric acid | Generator | | 2-Linoleoyl-1-palmitoyl-sn-glycero-3-phosphoric acid | Generator | | Phosphatidate(16:0/18:2) | Generator | | Phosphatidate(16:0/18:2OMEGA6) | Generator | | Phosphatidate(34:2) | Generator | | 1-Hexadecanoyl-2-(9Z,12Z-octadecadienoyl)-sn-phosphatidic acid | HMDB | | PA(16:0/18:2N6) | HMDB | | PA(16:0/18:2W6) | HMDB | | Phosphatidic acid(16:0/18:2n6) | HMDB | | Phosphatidic acid(16:0/18:2W6) | HMDB |
|
|---|
| Chemical Formula | C37H69O8P |
|---|
| Average Molecular Weight | 672.9127 |
|---|
| Monoisotopic Molecular Weight | 672.473005696 |
|---|
| IUPAC Name | [(2R)-3-(hexadecanoyloxy)-2-[(9Z,12Z)-octadeca-9,12-dienoyloxy]propoxy]phosphonic acid |
|---|
| Traditional Name | (2R)-3-(hexadecanoyloxy)-2-[(9Z,12Z)-octadeca-9,12-dienoyloxy]propoxyphosphonic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCCCCCCCCCCCCCCC(=O)OC[C@]([H])(COP(O)(=O)O)OC(=O)CCCCCCC\C=C/C\C=C/CCCCC |
|---|
| InChI Identifier | InChI=1S/C37H69O8P/c1-3-5-7-9-11-13-15-17-18-20-22-24-26-28-30-32-37(39)45-35(34-44-46(40,41)42)33-43-36(38)31-29-27-25-23-21-19-16-14-12-10-8-6-4-2/h11,13,17-18,35H,3-10,12,14-16,19-34H2,1-2H3,(H2,40,41,42)/b13-11-,18-17-/t35-/m1/s1 |
|---|
| InChI Key | YQMUIZXKIKXZHD-UMKNCJEQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,2-diacylglycerol-3-phosphates. These are glycerol-3-phosphates in which the glycerol moiety is bonded to two aliphatic chains through ester linkages. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphates |
|---|
| Direct Parent | 1,2-diacylglycerol-3-phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,2-diacylglycerol-3-phosphate
- Fatty acid ester
- Monoalkyl phosphate
- Dicarboxylic acid or derivatives
- Fatty acyl
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Carboxylic acid ester
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Intracellular membrane
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01ot-9472312000-5a0ace43ce559faf6b1a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-022l-0192214000-0ff5badab9b87707949e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01p2-1292111000-4a699460a7645a5a2ac2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03ds-0392011000-a011b8cc1a3b8c4d40a7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-05dr-3091102000-a4a318dd2dd7912c0151 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9060000000-f674e5442d2d2e19904c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9010000000-be58bb50c1230574e53c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000009000-6028356bb714d18e544d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-062f-1196606000-8572e410961d7f243448 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6r-0090200000-7fcea8001e422200fee2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ab9-0000009000-4c1c2d930aa8ce64404a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00fr-0000059000-4ff73b97ce6c9126eb82 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ou-0006693000-acbbee6d58a28139e3c9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0000009000-f21c401a141e9540f901 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0000099000-734455b81d5b79583fa4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kk-0000923000-1bab141842de7a393a0a | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| Pathways | |
|---|