| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 17:20:07 UTC |
|---|
| Update Date | 2020-05-11 18:45:02 UTC |
|---|
| BMDB ID | BMDB0008381 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | PC(20:3(5Z,8Z,11Z)/22:0) |
|---|
| Description | PC(20:3(5Z,8Z,11Z)/22:0), also known as pc(20:3(5z,8z,11z)/22:0) or pc(20:3(5z,8z,11z)/22:0), belongs to the class of organic compounds known as phosphatidylcholines. These are glycerophosphocholines in which the two free -OH are attached to one fatty acid each through an ester linkage. PC(20:3(5Z,8Z,11Z)/22:0) is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). PC(20:3(5Z,8Z,11Z)/22:0) participates in a number of enzymatic reactions, within cattle. In particular, S-Adenosylhomocysteine and PC(20:3(5Z,8Z,11Z)/22:0) can be biosynthesized from S-adenosylmethionine and pe-nme2(20:3(5Z,8Z,11Z)/22:0); which is catalyzed by the enzyme phosphatidylethanolamine N-methyltransferase. Furthermore, Cytidine monophosphate and PC(20:3(5Z,8Z,11Z)/22:0) can be biosynthesized from CDP-choline and DG(20:3(5Z,8Z,11Z)/22:0/0:0); which is mediated by the enzyme choline/ethanolaminephosphotransferase. Finally, PC(20:3(5Z,8Z,11Z)/22:0) and L-serine can be converted into choline and PS(20:3(5Z,8Z,11Z)/22:0) through the action of the enzyme phosphatidylserine synthase. In cattle, PC(20:3(5Z,8Z,11Z)/22:0) is involved in a couple of metabolic pathways, which include phosphatidylcholine biosynthesis PC(20:3(5Z,8Z,11Z)/22:0) pathway and phosphatidylethanolamine biosynthesis pe(20:3(5Z,8Z,11Z)/22:0) pathway. |
|---|
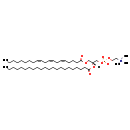
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Meadoyl-2-behenoyl-sn-glycero-3-phosphocholine | HMDB | | Phosphatidylcholine(20:3/22:0) | HMDB | | Phosphatidylcholine(42:3) | HMDB | | GPCho(42:3) | HMDB | | Lecithin | HMDB | | PC(20:3/22:0) | HMDB | | PC(42:3) | HMDB | | 1-(5Z,8Z,11Z-Eicosatrienoyl)-2-docosanoyl-sn-glycero-3-phosphocholine | HMDB | | GPCho(20:3/22:0) | HMDB | | PC(20:3(5Z,8Z,11Z)/22:0) | Lipid Annotator |
|
|---|
| Chemical Formula | C50H94NO8P |
|---|
| Average Molecular Weight | 868.257 |
|---|
| Monoisotopic Molecular Weight | 867.671705501 |
|---|
| IUPAC Name | (2-{[(2R)-2-(docosanoyloxy)-3-[(5Z,8Z,11Z)-icosa-5,8,11-trienoyloxy]propyl phosphonato]oxy}ethyl)trimethylazanium |
|---|
| Traditional Name | lecithin |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCCCCCCCCCCCCCCCCCCCCC(=O)O[C@]([H])(COC(=O)CCC\C=C/C\C=C/C\C=C/CCCCCCCC)COP([O-])(=O)OCC[N+](C)(C)C |
|---|
| InChI Identifier | InChI=1S/C50H94NO8P/c1-6-8-10-12-14-16-18-20-22-24-25-27-29-31-33-35-37-39-41-43-50(53)59-48(47-58-60(54,55)57-45-44-51(3,4)5)46-56-49(52)42-40-38-36-34-32-30-28-26-23-21-19-17-15-13-11-9-7-2/h21,23,28,30,34,36,48H,6-20,22,24-27,29,31-33,35,37-47H2,1-5H3/b23-21-,30-28-,36-34-/t48-/m1/s1 |
|---|
| InChI Key | HEKZKFWBJQHLFR-WKEFTTMRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phosphatidylcholines. These are glycerophosphocholines in which the two free -OH are attached to one fatty acid each through an ester linkage. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphocholines |
|---|
| Direct Parent | Phosphatidylcholines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diacylglycero-3-phosphocholine
- Phosphocholine
- Fatty acid ester
- Dialkyl phosphate
- Dicarboxylic acid or derivatives
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Fatty acyl
- Quaternary ammonium salt
- Tetraalkylammonium salt
- Carboxylic acid ester
- Carboxylic acid derivative
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Amine
- Organic salt
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Intracellular membrane
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000000090-772da7b866e9ed344511 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0159-0600000090-46517aad62e45a5fba71 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-1900041030-a21ef79557b05839bdf1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000000009-ef993d5bc5e7edc715ec | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0003000009-7f56ed1a069298c54d73 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-14ii-0009000004-8f60016247f16ea75d11 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0000000090-140e863d2d8d9d0b11d7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0000000090-beed4776ed98bf1b9402 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4k-0900161930-78de0d1a788067414291 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000000090-2a2c75387049677f0c6e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-06di-0017003090-97dbd266b8c3e9d16ef9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-5119300000-a8b47f58095d2b4d3296 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000000090-409bc7cb5bd9edcbbe9f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0000000090-f9045984e09e010968ce | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00l6-0100197030-50e6b6b09c9b47550884 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000000090-071e1880e207002eb2f4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0159-0600000090-f7423d0ba0e9a9849d1e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-1900041030-bee6a19604e7c41c9c63 | View in MoNA |
|---|
|
|---|