| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 17:42:33 UTC |
|---|
| Update Date | 2020-05-11 19:40:55 UTC |
|---|
| BMDB ID | BMDB0009426 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | PE(20:4(8Z,11Z,14Z,17Z)/18:4(6Z,9Z,12Z,15Z)) |
|---|
| Description | PE(20:4(8Z,11Z,14Z,17Z)/18:4(6Z,9Z,12Z,15Z)) is a phosphatidylethanolamine. It is a glycerophospholipid in which a phosphorylethanolamine moiety occupies a glycerol substitution site. As is the case with diacylglycerols, glycerophosphoethanolamines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 atoms. PE(20:4(8Z,11Z,14Z,17Z)/18:4(6Z,9Z,12Z,15Z)), in particular, consists of one 8Z,11Z,14Z,17Z-eicosapentaenoyl chain to the C-1 atom, and one 6Z,9Z,12Z,15Z-octadecatetraenoyl to the C-2 atom. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PEs are neutral zwitterions at physiological pH. They mostly have palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. PE synthesis can occur via two pathways. The first requires that ethanolamine be activated by phosphorylation and then coupled to CDP. The ethanolamine is then transferred from CDP-ethanolamine to phosphatidic acid to yield PE. The second involves the decarboxylation of PS. |
|---|
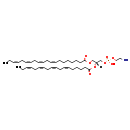
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Phophatidylethanolamine(20:4/18:4) | HMDB | | PE(38:8) | HMDB | | 1-Eicsoatetraenoyl-2-stearidonoyl-sn-glycero-3-phosphoethanolamine | HMDB | | PE(20:4/18:4) | HMDB | | GPEtn(38:8) | HMDB | | GPEtn(20:4/18:4) | HMDB | | 1-(8Z,11Z,14Z,17Z-Eicosapentaenoyl)-2-(6Z,9Z,12Z,15Z-octadecatetraenoyl)-sn-glycero-3-phosphoethanolamine | HMDB | | Phophatidylethanolamine(38:8) | HMDB | | PE(20:4(8Z,11Z,14Z,17Z)/18:4(6Z,9Z,12Z,15Z)) | Lipid Annotator |
|
|---|
| Chemical Formula | C43H70NO8P |
|---|
| Average Molecular Weight | 759.9916 |
|---|
| Monoisotopic Molecular Weight | 759.483904733 |
|---|
| IUPAC Name | (2-aminoethoxy)[(2R)-3-[(8Z,11Z,14Z,17Z)-icosa-8,11,14,17-tetraenoyloxy]-2-[(6Z,9Z,12Z,15Z)-octadeca-6,9,12,15-tetraenoyloxy]propoxy]phosphinic acid |
|---|
| Traditional Name | 2-aminoethoxy(2R)-3-[(8Z,11Z,14Z,17Z)-icosa-8,11,14,17-tetraenoyloxy]-2-[(6Z,9Z,12Z,15Z)-octadeca-6,9,12,15-tetraenoyloxy]propoxyphosphinic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@](COC(=O)CCCCCC\C=C/C\C=C/C\C=C/C\C=C/CC)(COP(O)(=O)OCCN)OC(=O)CCCC\C=C/C\C=C/C\C=C/C\C=C/CC |
|---|
| InChI Identifier | InChI=1S/C43H70NO8P/c1-3-5-7-9-11-13-15-17-19-20-22-23-25-27-29-31-33-35-42(45)49-39-41(40-51-53(47,48)50-38-37-44)52-43(46)36-34-32-30-28-26-24-21-18-16-14-12-10-8-6-4-2/h5-8,11-14,17-19,21-23,26,28,41H,3-4,9-10,15-16,20,24-25,27,29-40,44H2,1-2H3,(H,47,48)/b7-5-,8-6-,13-11-,14-12-,19-17-,21-18-,23-22-,28-26-/t41-/m1/s1 |
|---|
| InChI Key | RYYYESISLNMOCC-ADYFFADGSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phosphatidylethanolamines. These are glycerophosphoetahnolamines in which two fatty acids are bonded to the glycerol moiety through ester linkages. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphoethanolamines |
|---|
| Direct Parent | Phosphatidylethanolamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diacylglycero-3-phosphoethanolamine
- Phosphoethanolamine
- Fatty acid ester
- Dialkyl phosphate
- Dicarboxylic acid or derivatives
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Fatty acyl
- Amino acid or derivatives
- Carboxylic acid ester
- Carboxylic acid derivative
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Amine
- Primary aliphatic amine
- Organic nitrogen compound
- Primary amine
- Carbonyl group
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Intracellular membrane
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0011000900-51d0fc9cb15993129131 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0011000900-51d0fc9cb15993129131 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ufr-0399410600-075c8d7b1cde8908b195 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000001900-258072797f24bff0cbb4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-02t9-0003419700-5e3957756652ef95a1f3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0003419300-29b6a6020a5ca25ef505 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000001900-00d0b37fd153e95db980 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-02t9-0003419700-151d10507e29e575198a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0003419300-7d447f0afce78c09d7f3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0011000900-3a44b7209e31c3d67bea | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0011000900-3a44b7209e31c3d67bea | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ufr-0399410600-2a3eabee47502e01fb45 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0000001900-3b0b28ecd0c257ba9637 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0000001900-a53c1cd07c6b9b831b4f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0100201900-d65e88b85ab0dd137654 | View in MoNA |
|---|
|
|---|