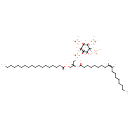
Showing metabocard for PIP3(18:0/18:1(9Z)) (BMDB0010153)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2016-10-03 18:00:50 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2020-05-11 19:50:53 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMDB ID | BMDB0010153 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | PIP3(18:0/18:1(9Z)) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | PIP3(18:0/18:1(9Z)) is an extremely weak basic (essentially neutral) compound (based on its pKa). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C45H88O22P4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight | 1105.072 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight | 1104.471772466 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | {[(1S,2S,3S)-2,4-dihydroxy-3-({hydroxy[(2R)-2-[(9Z)-octadec-9-enoyloxy]-3-(octadecanoyloxy)propoxy]phosphoryl}oxy)-5,6-bis(phosphonooxy)cyclohexyl]oxy}phosphonic acid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | [(1S,2S,3S)-2,4-dihydroxy-3-{[hydroxy((2R)-2-[(9Z)-octadec-9-enoyloxy]-3-(octadecanoyloxy)propoxy)phosphoryl]oxy}-5,6-bis(phosphonooxy)cyclohexyl]oxyphosphonic acid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | [H]\C(CCCCCCCC)=C(/[H])CCCCCCCC(=O)O[C@]([H])(COC(=O)CCCCCCCCCCCCCCCCC)COP(O)(=O)O[C@@]1([H])C([H])(O)C([H])(OP(O)(O)=O)C([H])(OP(O)(O)=O)[C@@]([H])(OP(O)(O)=O)[C@@]1([H])O | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C45H88O22P4/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-38(46)61-35-37(63-39(47)34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-4-2)36-62-71(59,60)67-42-40(48)43(64-68(50,51)52)45(66-70(56,57)58)44(41(42)49)65-69(53,54)55/h18,20,37,40-45,48-49H,3-17,19,21-36H2,1-2H3,(H,59,60)(H2,50,51,52)(H2,53,54,55)(H2,56,57,58)/b20-18-/t37-,40+,41?,42-,43+,44?,45?/m1/s1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | SGNSEVVFGCQOEF-MJHDCKEKSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Classification | Not classified | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ontology | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Expected but not Quantified | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofunction | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Application | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular locations |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biospecimen Locations |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phenol Explorer Compound ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FooDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KNApSAcK ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemspider ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Compound ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BiGG ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| METLIN ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in ARF GTPase activator activity
- Specific function:
- GTPase-activating protein (GAP) for ADP ribosylation factor 6 (ARF6) required for clathrin-dependent export of proteins from recycling endosomes to trans-Golgi network and cell surface. Required for regulated export of ITGB1 from recycling endosomes to the cell surface and ITGB1-dependent cell migration (By similarity).
- Gene Name:
- ACAP1
- Uniprot ID:
- A5PK26
- Molecular weight:
- 82129.0