| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:01:34 UTC |
|---|
| Update Date | 2020-04-22 15:39:28 UTC |
|---|
| BMDB ID | BMDB0010216 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 5,15-DiHETE |
|---|
| Description | 5,15-DiHETE, also known as 5S,15S-dihete, belongs to the class of organic compounds known as hydroxyeicosatetraenoic acids. These are eicosanoic acids with an attached hydroxyl group and four CC double bonds. Thus, 5,15-dihete is considered to be an eicosanoid. Based on a literature review a significant number of articles have been published on 5,15-DiHETE. |
|---|
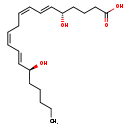
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (5S,15S)-Dihydroxy-(6E,8Z,11Z,13E)-eicosatetraenoic acid | ChEBI | | (5S,15S)-Dihydroxy-(6E,8Z,11Z,13E)-icosatetraenoic acid | ChEBI | | (5S,6E,8Z,11Z,13E,15S)-5,15-Dihydroxyicosatetraenoic acid | ChEBI | | 5(S),15(S)-Dihydroxy-6,13-trans-8,11-cis-eicosatetraenoic acid | ChEBI | | 5,15-Dihydroxy-6,8,11,13-eicosatetraenoic acid | ChEBI | | 5,15-Dihydroxyeicosatetraenoic acid | ChEBI | | 5S,15S-DiHETE | ChEBI | | 5S,15S-Dihydroxy-6E,8Z,11Z,13E-eicosatetraenoic acid | ChEBI | | (5S,15S)-Dihydroxy-(6E,8Z,11Z,13E)-eicosatetraenoate | Generator | | (5S,15S)-Dihydroxy-(6E,8Z,11Z,13E)-icosatetraenoate | Generator | | (5S,6E,8Z,11Z,13E,15S)-5,15-Dihydroxyicosatetraenoate | Generator | | 5(S),15(S)-Dihydroxy-6,13-trans-8,11-cis-eicosatetraenoate | Generator | | 5,15-Dihydroxy-6,8,11,13-eicosatetraenoate | Generator | | 5,15-Dihydroxyeicosatetraenoate | Generator | | 5S,15S-Dihydroxy-6E,8Z,11Z,13E-eicosatetraenoate | Generator | | 5(S),15(S)-DiHETE | HMDB | | 5,15-DiHETE | ChEBI |
|
|---|
| Chemical Formula | C20H32O4 |
|---|
| Average Molecular Weight | 336.4657 |
|---|
| Monoisotopic Molecular Weight | 336.230059512 |
|---|
| IUPAC Name | (5S,6E,8Z,11Z,13E,15S)-5,15-dihydroxyicosa-6,8,11,13-tetraenoic acid |
|---|
| Traditional Name | 5S,15S-DiHETE |
|---|
| CAS Registry Number | 82200-87-1 |
|---|
| SMILES | CCCCC[C@H](O)\C=C\C=C/C\C=C/C=C/[C@@H](O)CCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C20H32O4/c1-2-3-9-13-18(21)14-10-7-5-4-6-8-11-15-19(22)16-12-17-20(23)24/h5-8,10-11,14-15,18-19,21-22H,2-4,9,12-13,16-17H2,1H3,(H,23,24)/b7-5-,8-6-,14-10+,15-11+/t18-,19+/m0/s1 |
|---|
| InChI Key | UXGXCGPWGSUMNI-BVHTXILBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroxyeicosatetraenoic acids. These are eicosanoic acids with an attached hydroxyl group and four CC double bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Eicosanoids |
|---|
| Direct Parent | Hydroxyeicosatetraenoic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydroxyeicosatetraenoic acid
- Long-chain fatty acid
- Hydroxy fatty acid
- Fatty acid
- Unsaturated fatty acid
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic oxide
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Carbonyl group
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00kk-6492000000-3c2ee47ee6c0da2b1c18 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-002u-9013440000-ef35dbcf7f2fcff2741e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0gb9-0019000000-f48b85306f04941fd350 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-106r-4196000000-2bead0468d6c53e9925e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0596-9460000000-5fbe33b7844341ee0d01 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00kr-0029000000-d8e68d87d5e32a76be4d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01bi-3069000000-b36c2c202ba716d4d1b9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9040000000-45f4070691efc333f188 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uxr-0019000000-59a1f0edad1bc877238e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-2449000000-fb935c85ebd7e2a9b21b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014m-9620000000-f3e46e59b0649a6e56bc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00kr-0019000000-c96c619b8b59ea16cad1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014r-3289000000-317e4e3f86ab790bd361 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-07be-8291000000-9698a4ac42a43db4361b | View in MoNA |
|---|
|
|---|