| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:04:32 UTC |
|---|
| Update Date | 2020-05-11 20:25:05 UTC |
|---|
| BMDB ID | BMDB0010333 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Estriol-17-glucuronide |
|---|
| Description | Estriol-17-glucuronide belongs to the class of organic compounds known as steroidal glycosides. These are sterol lipids containing a carbohydrate moiety glycosidically linked to the steroid skeleton. Thus, estriol-17-glucuronide is considered to be a steroid conjugate. Based on a literature review very few articles have been published on Estriol-17-glucuronide. |
|---|
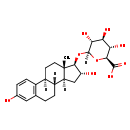
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (16alpha,17beta)-3,16-Dihydroxyestra-1,3,5(10)-trien-17-yl beta-D-glucopyranosiduronic acid | HMDB | | (16alpha,17beta)-3,16-Dihydroxyestra-1,3,5(10)-trien-17-yl beta-delta-glucopyranosiduronic acid | HMDB | | 17beta-(beta-D-Glucopyranuronosyloxy)estra-1,3,5(10)-triene-3,16alpha-diol | HMDB | | 17beta-(beta-delta-Glucopyranuronosyloxy) estra-1,3,5(10)-triene-3,16alpha-diol | HMDB | | 17beta-(beta-delta-Glucopyranuronosyloxy)estra-1,3,5(10)-triene-3,16alpha-diol | HMDB | | 3,16alpha-Dihydroxyestra-1,3,5(10)-trien-17beta-yl glucopyranosiduronic acid | HMDB | | beta-D-Glucopyranosiduronic acid derivative OF estrane | HMDB | | beta-delta-Glucopyranosiduronic acid derivative OF estrane | HMDB | | Estriol 17-glucuronide | HMDB | | Estriol 17beta-(beta-D-glucuronide) | HMDB | | Estriol 17beta-glucuronide | HMDB | | Estriol 17beta-monoglucuronide | HMDB | | 16-a,17-b-Estriol 17-b-D-glucuronide | HMDB | | 16-Α,17-β-estriol 17-β-D-glucuronide | HMDB | | Estriol 17β-(β-D-glucuronide) | HMDB | | Estriol 17beta-(β-D-glucuronide) | HMDB | | 16Α,17β-estriol 17-(β-D-glucuronide) | HMDB | | 16alpha,17beta-Estriol 17-(beta-D-glucuronide) | HMDB | | 16-alpha,17-beta-Estriol 17-beta-D-glucuronide | HMDB |
|
|---|
| Chemical Formula | C24H32O9 |
|---|
| Average Molecular Weight | 464.511 |
|---|
| Monoisotopic Molecular Weight | 464.20463261 |
|---|
| IUPAC Name | (2S,3S,4S,5R,6R)-6-{[(1S,10R,11S,13R,14R,15S)-5,13-dihydroxy-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-trien-14-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| Traditional Name | (2S,3S,4S,5R,6R)-6-{[(1S,10R,11S,13R,14R,15S)-5,13-dihydroxy-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-trien-14-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| CAS Registry Number | 7219-89-8 |
|---|
| SMILES | [H][C@@]12C[C@@H](O)[C@H](O[C@]3([H])O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)[C@@]1(C)CC[C@]1([H])C3=C(CC[C@@]21[H])C=C(O)C=C3 |
|---|
| InChI Identifier | InChI=1S/C24H32O9/c1-24-7-6-13-12-5-3-11(25)8-10(12)2-4-14(13)15(24)9-16(26)21(24)33-23-19(29)17(27)18(28)20(32-23)22(30)31/h3,5,8,13-21,23,25-29H,2,4,6-7,9H2,1H3,(H,30,31)/t13-,14-,15+,16-,17+,18+,19-,20+,21+,23+,24+/m1/s1 |
|---|
| InChI Key | CZGFLAQOJPXVRV-FLVROIOLSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as steroidal glycosides. These are sterol lipids containing a carbohydrate moiety glycosidically linked to the steroid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroidal glycosides |
|---|
| Direct Parent | Steroidal glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Steroidal glycoside
- Estrogen-skeleton
- 3-hydroxysteroid
- Estrane-skeleton
- Hydroxysteroid
- 16-alpha-hydroxysteroid
- 16-hydroxysteroid
- 1-o-glucuronide
- O-glucuronide
- Phenanthrene
- Glucuronic acid or derivatives
- Hexose monosaccharide
- O-glycosyl compound
- Glycosyl compound
- Tetralin
- Beta-hydroxy acid
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Pyran
- Oxane
- Monosaccharide
- Hydroxy acid
- Cyclic alcohol
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Carboxylic acid derivative
- Carboxylic acid
- Acetal
- Monocarboxylic acid or derivatives
- Alcohol
- Organooxygen compound
- Organic oxygen compound
- Organic oxide
- Carbonyl group
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000900000-5761e9c280d344cbe1d1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kr-2391400000-32101bfa4a41b480ecfc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kr-4290000000-ad36d69ddfc50e9db602 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00ks-0090800000-40ebbed6c09f66d32278 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0079-0390100000-583e7fea563381217514 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-022i-0590000000-ac0dc1bbdde5286b36d4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00rj-0080900000-20b5a9f6cd51b3fec414 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0190200000-69ce9a6bcc77fafb7d43 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-0941000000-6eb143c59b94907725f6 | View in MoNA |
|---|
|
|---|