| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:04:35 UTC |
|---|
| Update Date | 2020-05-11 20:25:07 UTC |
|---|
| BMDB ID | BMDB0010335 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Estriol-3-glucuronide |
|---|
| Description | Estriol-3-glucuronide, also known as estriol 3-glucosiduronate, belongs to the class of organic compounds known as steroid glucuronide conjugates. These are sterol lipids containing a glucuronide moiety linked to the steroid skeleton. Thus, estriol-3-glucuronide is considered to be a steroid conjugate lipid molecule. Estriol-3-glucuronide is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. |
|---|
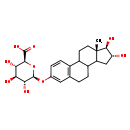
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2S,3S,4S,5R,6S)-6-[[(13S,16R,17R)-16,17-Dihydroxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-3-yl]oxy]-3,4,5-trihydroxyoxane-2-carboxylic acid | HMDB | | 16a,17b-Dihydroxyestra-1,3,5(10)-trien-3-yl-beta-D-glucosiduronic acid | HMDB | | 16a,17b-Dihydroxyestra-1,3,5(10)-trien-3-yl-beta-delta-glucosiduronic acid | HMDB | | 16a,17b-Dihydroxyestra-1,3,5(10)-trien-3-yl-glucopyranosiduronic acid | HMDB | | 16alpha,17beta-Dihydroxyestra-1,3,5(10)-trien-3-yl-beta-D-glucosiduronic acid | HMDB | | 16alpha,17beta-Dihydroxyestra-1,3,5(10)-trien-3-yl-beta-delta-glucosiduronic acid | HMDB | | 16alpha,17beta-Dihydroxyestra-1,3,5(10)-trien-3-yl-glucopyranosiduronic acid | HMDB | | Estriol 3-(beta-D-glucuronide) | HMDB | | Estriol 3-(beta-delta-glucuronide) | HMDB | | Estriol 3-beta-glucuronide | HMDB | | Estriol 3-glucopyranuronoside | HMDB | | Estriol 3-glucosiduronate | HMDB | | Estriol 3-glucuronoside | HMDB | | Estriol 3-monoglucuronide | HMDB | | Estriol 3-glucuronide | HMDB | | Estriol 3-glucuronide, (16beta,17beta)-isomer | HMDB | | Estriol 3-glucuronide, monosodium salt | HMDB | | Estriol 3-glucuronide, monosodium salt, 2,4,17-(2)H-labeled | HMDB | | Estriol 3-glucuronide, (16alpha,17alpha)-isomer | HMDB |
|
|---|
| Chemical Formula | C24H32O9 |
|---|
| Average Molecular Weight | 464.5055 |
|---|
| Monoisotopic Molecular Weight | 464.204632622 |
|---|
| IUPAC Name | (2S,3S,4S,5R,6S)-6-{[(13R,14R,15S)-13,14-dihydroxy-15-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-2(7),3,5-trien-5-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| Traditional Name | (2S,3S,4S,5R,6S)-6-{[(13R,14R,15S)-13,14-dihydroxy-15-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-2(7),3,5-trien-5-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| CAS Registry Number | 2479-91-6 |
|---|
| SMILES | C[C@]12CCC3C(CCC4=C3C=CC(O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)=C4)C1C[C@@H](O)[C@@H]2O |
|---|
| InChI Identifier | InChI=1S/C24H32O9/c1-24-7-6-13-12-5-3-11(32-23-19(28)17(26)18(27)20(33-23)22(30)31)8-10(12)2-4-14(13)15(24)9-16(25)21(24)29/h3,5,8,13-21,23,25-29H,2,4,6-7,9H2,1H3,(H,30,31)/t13?,14?,15?,16-,17+,18+,19-,20+,21+,23-,24+/m1/s1 |
|---|
| InChI Key | UZKIAJMSMKLBQE-WTSDUJKYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as steroid glucuronide conjugates. These are sterol lipids containing a glucuronide moiety linked to the steroid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroidal glycosides |
|---|
| Direct Parent | Steroid glucuronide conjugates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Steroid-glucuronide-skeleton
- Estrane-skeleton
- Hydroxysteroid
- 16-alpha-hydroxysteroid
- 16-hydroxysteroid
- 17-hydroxysteroid
- Fatty acyl glycoside
- Fatty acyl glycoside of mono- or disaccharide
- Phenolic glycoside
- Phenanthrene
- O-glucuronide
- 1-o-glucuronide
- Glucuronic acid or derivatives
- Alkyl glycoside
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Tetralin
- Beta-hydroxy acid
- Benzenoid
- Fatty acyl
- Pyran
- Oxane
- Monosaccharide
- Hydroxy acid
- Cyclic alcohol
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Polyol
- Acetal
- Organoheterocyclic compound
- Carboxylic acid
- Carboxylic acid derivative
- Oxacycle
- Organooxygen compound
- Alcohol
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0012-3003900000-3a759fac05f0b13014fe | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-014i-2111069000-aa834adc6c9496013e47 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00rj-0190800000-d1c6fc118050f296177e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dr-0290100000-f9cd4d37e0002e8d2d7d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dr-0690000000-fe172a1edd7eabc9e795 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03y0-1240900000-541a32d4a20df007f92e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kr-1290300000-42261425fa6d65092982 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-3190000000-8366728f76e8fb479901 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0063900000-72267838e3bc10796ac6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0670-0225900000-c023ca158db64dbf7a17 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05tf-0953300000-6284210872a751951cf0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0100900000-58bd2cdf71264bfe1c81 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kr-1090200000-46db53c3915f95a654c2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052r-5091200000-163c1340ff01e125474d | View in MoNA |
|---|
|
|---|