| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:12:28 UTC |
|---|
| Update Date | 2020-05-21 16:27:14 UTC |
|---|
| BMDB ID | BMDB0010718 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | (R)-3-Hydroxyhexanoic acid |
|---|
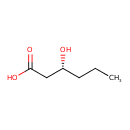
| Description | (R)-3-Hydroxyhexanoic acid belongs to the class of organic compounds known as medium-chain hydroxy acids and derivatives. These are hydroxy acids with a 6 to 12 carbon atoms long side chain (R)-3-Hydroxyhexanoic acid is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral (R)-3-Hydroxyhexanoic acid exists in all eukaryotes, ranging from yeast to humans (R)-3-Hydroxyhexanoic acid participates in a number of enzymatic reactions, within cattle. In particular, (R)-3-Hydroxyhexanoic acid can be biosynthesized from 3-oxohexanoic acid; which is catalyzed by the enzyme fatty acid synthase. Beta ketoacyl synthase domain. In addition, (R)-3-Hydroxyhexanoic acid can be converted into trans-hex-2-enoic acid through the action of the enzyme fatty acid synthase. dyhydrase domain. In cattle, (R)-3-hydroxyhexanoic acid is involved in the metabolic pathway called fatty acid biosynthesis pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (R)-3-Hydroxyhexanoate | Generator | | (3R)-3-Hydroxyhexanoate | HMDB | | (3R)-3-Hydroxyhexanoic acid | HMDB | | (±)-3-hydroxyhexanoate | HMDB | | (±)-3-hydroxyhexanoic acid | HMDB | | 3-Hydroxycaproate | HMDB | | 3-Hydroxycaproic acid | HMDB | | 3-Hydroxyhexanoate | HMDB | | 3-Hydroxyhexanoic acid | HMDB | | D-beta-Hydroxycaproate | HMDB | | D-beta-Hydroxycaproic acid | HMDB | | D-Β-hydroxycaproate | HMDB | | D-Β-hydroxycaproic acid | HMDB | | FA(6:0(3-OH)) | HMDB | | FA(6:0(3R-OH)) | HMDB | | beta-Hydroxy-N-caproate | HMDB | | beta-Hydroxy-N-caproic acid | HMDB | | beta-Hydroxycaproate | HMDB | | beta-Hydroxycaproic acid | HMDB | | beta-Hydroxyhexanoate | HMDB | | beta-Hydroxyhexanoic acid | HMDB | | Β-hydroxy-N-caproate | HMDB | | Β-hydroxy-N-caproic acid | HMDB | | Β-hydroxycaproate | HMDB | | Β-hydroxycaproic acid | HMDB | | Β-hydroxyhexanoate | HMDB | | Β-hydroxyhexanoic acid | HMDB | | (R)-3-Hydroxyhexanoic acid | HMDB |
|
|---|
| Chemical Formula | C6H12O3 |
|---|
| Average Molecular Weight | 132.1577 |
|---|
| Monoisotopic Molecular Weight | 132.07864425 |
|---|
| IUPAC Name | (3R)-3-hydroxyhexanoic acid |
|---|
| Traditional Name | 3R-hydroxy-hexanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCC[C@@H](O)CC(O)=O |
|---|
| InChI Identifier | InChI=1S/C6H12O3/c1-2-3-5(7)4-6(8)9/h5,7H,2-4H2,1H3,(H,8,9)/t5-/m1/s1 |
|---|
| InChI Key | HPMGFDVTYHWBAG-RXMQYKEDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as medium-chain hydroxy acids and derivatives. These are hydroxy acids with a 6 to 12 carbon atoms long side chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Hydroxy acids and derivatives |
|---|
| Sub Class | Medium-chain hydroxy acids and derivatives |
|---|
| Direct Parent | Medium-chain hydroxy acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Medium-chain hydroxy acid
- Medium-chain fatty acid
- Beta-hydroxy acid
- Hydroxy fatty acid
- Fatty acyl
- Fatty acid
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic oxide
- Alcohol
- Carbonyl group
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9000000000-5263808e33ccb6565b3c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-009i-9330000000-5a1f5b24235b7a70faba | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0159-5900000000-16b15ce70244c2b64d0c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014j-9300000000-3cfe9ed23a846d781f37 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-b3878b3c92a65ddc51fd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-5900000000-ae1b58fe367907c50ffe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05nr-9300000000-f5a6c18cc1d3fca5f109 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05mo-9000000000-7911aea05314b944272c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0avi-9100000000-82b86020a271d61ba1b5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052f-9000000000-5b86d65468321ff15426 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-ab813e477e382c5c6c08 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-053r-6900000000-605bc451889b03fcac77 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4l-9100000000-e58c13d9ebd5ab0dbbbf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-191e4c2a7ad7ea5541dd | View in MoNA |
|---|
|
|---|