| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:12:35 UTC |
|---|
| Update Date | 2020-05-21 16:27:15 UTC |
|---|
| BMDB ID | BMDB0010725 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
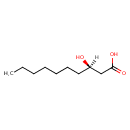
| Common Name | (R)-3-Hydroxydecanoic acid |
|---|
| Description | (R)-3-Hydroxydecanoic acid, also known as (r)-(r)-(r)-3-hydroxydecanoic acid or (r)-3-hydroxydecanoic acid, belongs to the class of organic compounds known as medium-chain hydroxy acids and derivatives. These are hydroxy acids with a 6 to 12 carbon atoms long side chain (R)-3-Hydroxydecanoic acid is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral (R)-3-Hydroxydecanoic acid exists in all eukaryotes, ranging from yeast to humans (R)-3-Hydroxydecanoic acid participates in a number of enzymatic reactions, within cattle. In particular, (R)-3-Hydroxydecanoic acid can be biosynthesized from 3-oxodecanoic acid through the action of the enzyme fatty acid synthase. Beta ketoacyl synthase domain. In addition, (R)-3-Hydroxydecanoic acid can be converted into trans-dec-2-enoic acid through the action of the enzyme fatty acid synthase. dyhydrase domain. In cattle, (R)-(r)-(r)-3-hydroxydecanoic acid is involved in the metabolic pathway called fatty acid biosynthesis pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (R)-3-Hydroxydecanoate | Generator | | 3-HDA | HMDB | | 3-Hydroxydecanoic acid | HMDB | | beta-Hydroxydecanoic acid | HMDB | | Myrmicacin, (+-)-isomer | HMDB | | Myrmicacin monosodium (+-)-isomer | HMDB | | 3-Hydroxy-decanoic acid | HMDB | | Myrmicacin, (R)-isomer | HMDB | | Myrmicacin | HMDB |
|
|---|
| Chemical Formula | C10H20O3 |
|---|
| Average Molecular Weight | 188.264 |
|---|
| Monoisotopic Molecular Weight | 188.141244506 |
|---|
| IUPAC Name | (3R)-3-hydroxydecanoic acid |
|---|
| Traditional Name | (R)-3-hydroxydecanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@](O)(CCCCCCC)CC(O)=O |
|---|
| InChI Identifier | InChI=1S/C10H20O3/c1-2-3-4-5-6-7-9(11)8-10(12)13/h9,11H,2-8H2,1H3,(H,12,13)/t9-/m1/s1 |
|---|
| InChI Key | FYSSBMZUBSBFJL-SECBINFHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as medium-chain hydroxy acids and derivatives. These are hydroxy acids with a 6 to 12 carbon atoms long side chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Hydroxy acids and derivatives |
|---|
| Sub Class | Medium-chain hydroxy acids and derivatives |
|---|
| Direct Parent | Medium-chain hydroxy acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Medium-chain hydroxy acid
- Medium-chain fatty acid
- Beta-hydroxy acid
- Hydroxy fatty acid
- Fatty acyl
- Fatty acid
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic oxide
- Alcohol
- Carbonyl group
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-007c-9200000000-f9795d95896f66d90766 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-010c-9041000000-b9b51f3624f22e4b6c9d | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dr-0900000000-1d9eae6ad3d5e431996b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fmr-3900000000-bbd0b333dda24219033a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9200000000-7cc23eacacf566b10ff0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-1900000000-46d09f9537cea04f6e13 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-054x-3900000000-76f4dd284af0c499a0a8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6u-9400000000-84da3410c532bb2e2273 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-1900000000-2019592125745914c6e0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a6r-9800000000-b6a04af2348e32c41166 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9000000000-8f7fd17dc3ad7497c377 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-009i-9800000000-2661614f9f11754f0d3a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4l-9000000000-b7cbaa25fb90f53def32 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9000000000-6609ad3e1fed5bc9c4ca | View in MoNA |
|---|
|
|---|