| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:12:46 UTC |
|---|
| Update Date | 2020-05-21 16:27:15 UTC |
|---|
| BMDB ID | BMDB0010735 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Trans-Hexa-dec-2-enoic acid |
|---|
| Description | trans-Hexa-dec-2-enoic acid, also known as (2E)-trans-hexa-dec-2-enoic acid or Trans-hexa-dec-2-enoic acid, belongs to the class of organic compounds known as long-chain fatty acids. These are fatty acids with an aliphatic tail that contains between 13 and 21 carbon atoms. trans-Hexa-dec-2-enoic acid is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. trans-Hexa-dec-2-enoic acid exists in all eukaryotes, ranging from yeast to humans. trans-Hexa-dec-2-enoic acid participates in a number of enzymatic reactions, within cattle. In particular, trans-Hexa-dec-2-enoic acid can be biosynthesized from (R)-3-hydroxy-hexadecanoic acid through the action of the enzyme fatty acid synthase. dyhydrase domain. In addition, trans-Hexa-dec-2-enoic acid can be converted into palmitic acid through the action of the enzyme fatty acid synthase. enoyl reductase domain. In cattle, trans-hexa-dec-2-enoic acid is involved in the metabolic pathway called fatty acid biosynthesis pathway. |
|---|
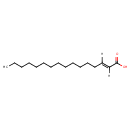
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| trans-Hexa-dec-2-enoate | Generator | | (2E)-Hexadecenoic acid | ChEBI | | (e)-2-Hexadecenoic acid | ChEBI | | 2-Palmitoleic acid | ChEBI | | 2-trans-Hexadecenoic acid | ChEBI | | Gaidic acid | ChEBI | | t-16:1D2 | ChEBI | | t-2-Hexadecenoic acid | ChEBI | | trans-2-Hexadecenoic acid | ChEBI | | trans-Delta(2)-Hexadecenoic acid | ChEBI | | trans-Hexadec-2-enoic acid | ChEBI | | (2E)-Hexadecenoate | Generator | | (e)-2-Hexadecenoate | Generator | | 2-Palmitoleate | Generator | | 2-trans-Hexadecenoate | Generator | | Gaidate | Generator | | t-2-Hexadecenoate | Generator | | trans-2-Hexadecenoate | Generator | | trans-delta(2)-Hexadecenoate | Generator | | trans-δ(2)-hexadecenoate | Generator | | trans-δ(2)-hexadecenoic acid | Generator | | trans-Hexadec-2-enoate | Generator | | Hexadecenoic acid, (e)-isomer | MeSH, HMDB | | cis-Hexadecenoic acid | MeSH, HMDB | | Hexadecenoic acid | MeSH, HMDB | | Hexadecenoic acid, (Z)-isomer | MeSH, HMDB | | trans-Hexadecenoic acid | MeSH, HMDB |
|
|---|
| Chemical Formula | C16H30O2 |
|---|
| Average Molecular Weight | 254.4082 |
|---|
| Monoisotopic Molecular Weight | 254.224580204 |
|---|
| IUPAC Name | (2E)-hexadec-2-enoic acid |
|---|
| Traditional Name | hexadecenoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H]\C(CCCCCCCCCCCCC)=C(\[H])C(O)=O |
|---|
| InChI Identifier | InChI=1S/C16H30O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16(17)18/h14-15H,2-13H2,1H3,(H,17,18)/b15-14+ |
|---|
| InChI Key | ZVRMGCSSSYZGSM-CCEZHUSRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as long-chain fatty acids. These are fatty acids with an aliphatic tail that contains between 13 and 21 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Long-chain fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Long-chain fatty acid
- Unsaturated fatty acid
- Straight chain fatty acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Adiposome
- Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 TMS) | splash10-017j-5900000000-58442a5c08ea8c4926d0 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-017j-5900000000-58442a5c08ea8c4926d0 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01p6-9820000000-ca98f4a0dd8ff557bc6c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-022i-9551000000-39e4b8ad34555c61b4ad | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0190000000-1228a4503bc27bed1ebf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4l-3960000000-1370ead704dcac4cec1f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9700000000-85e6708e40be2971ffc9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-bb3d892e7f48a711d0ce | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zfr-0090000000-6a46dea6de3429b73374 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9440000000-668a65146f3042b031de | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-37cfc189faecac3c9577 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-b570c7cdc86145fba021 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00n0-1960000000-8371cc75a9113569270b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-4490000000-61560c3648170efe9274 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ab9-9520000000-26416932e2aa67751576 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aor-9100000000-9eed9d946622d4a4c83c | View in MoNA |
|---|
|
|---|