| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:20:33 UTC |
|---|
| Update Date | 2020-05-11 19:18:27 UTC |
|---|
| BMDB ID | BMDB0011550 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | MG(0:0/20:5(5Z,8Z,11Z,14Z,17Z)/0:0) |
|---|
| Description | MG(0:0/20:5(5Z,8Z,11Z,14Z,17Z)/0:0) belongs to the family of monoradyglycerols, which are glycerolipids lipids containing a common glycerol backbone to which at one fatty acyl group is attached. Their general formula is [R1]OCC(CO[R2])O[R3]. MG(0:0/20:5(5Z,8Z,11Z,14Z,17Z)/0:0) is made up of one 5Z,8Z,11Z,14Z,17Z-eicosapentaenoyl(R2). |
|---|
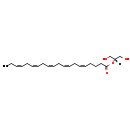
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Eicosapentaenoyl-glycerol | ChEBI | | MG(0:0/20:5(5Z,8Z,11Z,14Z,17Z)/0:0) | ChEBI |
|
|---|
| Chemical Formula | C23H36O4 |
|---|
| Average Molecular Weight | 376.5295 |
|---|
| Monoisotopic Molecular Weight | 376.26135964 |
|---|
| IUPAC Name | 1,3-dihydroxypropan-2-yl (5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaenoate |
|---|
| Traditional Name | 1,3-dihydroxypropan-2-yl (5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaenoate |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H]C(CO)(CO)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CC |
|---|
| InChI Identifier | InChI=1S/C23H36O4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-23(26)27-22(20-24)21-25/h3-4,6-7,9-10,12-13,15-16,22,24-25H,2,5,8,11,14,17-21H2,1H3/b4-3-,7-6-,10-9-,13-12-,16-15- |
|---|
| InChI Key | IXSYVBCFIGEECR-JLNKQSITSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as endocannabinoids. These are arachidonic acid derivatives containing either an N-acetylethanolamine(type 1) or a glycerol(types 2 and 3) moiety attached to the aliphatic tail. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Endocannabinoids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Endocannabinoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2-arachidonoylglycerol-skeleton
- 2-acyl-sn-glycerol
- Monoradylglycerol
- Monoacylglycerol
- Glycerolipid
- Fatty acid ester
- Fatty acyl
- Carboxylic acid ester
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Primary alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Organooxygen compound
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-056r-5192000000-e360545d382af40b962e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0q4r-4290020000-b5e72e4eff78a9db1ffd | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-8069000000-472947ead6054d40d7c7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0573-9262000000-a033ad020cd0bff33ab9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-054o-9270000000-562a7861d3af8ebf320f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0fi0-5019000000-567faba551a94213e8ce | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0596-9024000000-08383936180575baf90a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0abc-9021000000-7a357c29265b0ca4f8ed | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0009000000-4909244a4bab0f29e0df | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0009000000-4909244a4bab0f29e0df | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004p-0009000000-e2b3e56f7899533b89c1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0009000000-be499a6325a46761dae2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004l-0009000000-a454b093a1aeada19b3a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00i0-0009000000-4b6e61f199be154609bb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0009000000-41dad2bd4e1b27575660 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ui0-0009000000-c0fdef2a670eb5c23f30 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zg0-0039000000-cd3454365fc266ab63a8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00b9-6019000000-97bcbb62d035130ba2f7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0piu-9023000000-f51e7a68113ca07c5deb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pbi-9261000000-9651964e8c89663cc986 | View in MoNA |
|---|
|
|---|