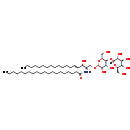| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:21:25 UTC |
|---|
| Update Date | 2020-04-22 15:45:30 UTC |
|---|
| BMDB ID | BMDB0011593 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Lactosylcermide (d18:1/20:0) |
|---|
| Description | Lactosylcermide (d18:1/20:0), also known as laccer(D18:1/20:0) or C20 lactosyl ceramide, belongs to the class of organic compounds known as 6-aminopurines. These are purines that carry an amino group at position 6. Purine is a bicyclic aromatic compound made up of a pyrimidine ring fused to an imidazole ring. Lactosylcermide (d18:1/20:0) is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| LacCer(d18:1/20:0) | ChEBI | | N-(Eicosanoyl)-1-b-lactosyl-sphing-4-enine | ChEBI | | 1-O-(4-O-b-D-Galactopyranosyl-b-D-glucopyranosyl)-ceramide | HMDB | | 1-O-(4-O-beta-D-Galactopyranosyl-beta-glucopyranosyl)ceramide | HMDB | | 1-O-(4-O-beta-D-Galactopyranosyl-beta-D-glucopyranosyl)-ceramide | HMDB | | beta-D-Galactosyl-1,4-beta-D-glucosylceramide | HMDB | | beta-D-Galactosyl-1,4-beta-D-glucosramide | HMDB | | C20 Lactosyl ceramide | HMDB | | CDH | HMDB | | CDW17 Antigen | HMDB | | Cytolipin H | HMDB | | D-Galactosyl-1,4-beta-D-glucosylceramide | HMDB | | D-Galactosyl-1-beta-D-glucosylceramide | HMDB | | Gal-beta1->4GLC-beta1->1'cer | HMDB | | LacC,4-beta-D-glucosylceramide | HMDB | | LacCer | HMDB | | Lactosyl ceramide (d18:1/20:0) | HMDB | | Lactosyl-N-acylsphingosine | HMDB | | Lactosylceramide | HMDB | | N-(Eicosanoyl)-1-beta-lactosyl-sphing-4-enine | HMDB | | N-(Hexadecanoyl)-1-b-lactosyl-sphing-4-enine | HMDB | | N-(Hexadecanoyl)-1-beta-lactosyl-sphing-4-enine | HMDB | | b-D-Galactosyl-(1->4)-b-D-glucosyl-(11)-N-eicosanoylsphingosine | Generator, HMDB | | β-D-Galactosyl-(1->4)-β-D-glucosyl-(11)-N-eicosanoylsphingosine | Generator, HMDB | | LacCer d18:1/20:0 | HMDB | | Lactosylceramide (d18:1,C20:0) | HMDB | | Lactosylceramide (d18:1/20:0) | HMDB | | Lactosylceramide(d18:1/20:0) | HMDB |
|
|---|
| Chemical Formula | C50H95NO13 |
|---|
| Average Molecular Weight | 918.2882 |
|---|
| Monoisotopic Molecular Weight | 917.680342131 |
|---|
| IUPAC Name | N-[(2S,3R,4E)-1-{[(2R,3R,4R,5S,6R)-3,4-dihydroxy-6-(hydroxymethyl)-5-{[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-2-yl]oxy}-3-hydroxyoctadec-4-en-2-yl]icosanamide |
|---|
| Traditional Name | N-[(2S,3R,4E)-1-{[(2R,3R,4R,5S,6R)-3,4-dihydroxy-6-(hydroxymethyl)-5-{[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-2-yl]oxy}-3-hydroxyoctadec-4-en-2-yl]icosanamide |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@](CO[C@@H]1O[C@H](CO)[C@@H](O[C@@H]2O[C@H](CO)[C@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O)(NC(=O)CCCCCCCCCCCCCCCCCCC)[C@]([H])(O)\C=C\CCCCCCCCCCCCC |
|---|
| InChI Identifier | InChI=1S/C50H95NO13/c1-3-5-7-9-11-13-15-17-18-19-20-22-24-26-28-30-32-34-42(55)51-38(39(54)33-31-29-27-25-23-21-16-14-12-10-8-6-4-2)37-61-49-47(60)45(58)48(41(36-53)63-49)64-50-46(59)44(57)43(56)40(35-52)62-50/h31,33,38-41,43-50,52-54,56-60H,3-30,32,34-37H2,1-2H3,(H,51,55)/b33-31+/t38-,39+,40+,41+,43-,44-,45+,46+,47+,48+,49+,50-/m0/s1 |
|---|
| InChI Key | FGJIXPPBZNPEHW-CRMMDXJYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 6-aminopurines. These are purines that carry an amino group at position 6. Purine is a bicyclic aromatic compound made up of a pyrimidine ring fused to an imidazole ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Imidazopyrimidines |
|---|
| Sub Class | Purines and purine derivatives |
|---|
| Direct Parent | 6-aminopurines |
|---|
| Alternative Parents | |
|---|
| Substituents | - 6-aminopurine
- Aminopyrimidine
- Pyrimidine
- Heteroaromatic compound
- Imidazole
- Azole
- Azacycle
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Primary amine
- Organonitrogen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Endosome
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0zg0-0130032928-7752769383ae73c606b8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002r-0140161911-2533e445ce9facf69eb6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03y0-1691731841-9436dcfc457ff307f07c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0gi1-1532052978-5226462fdd198982b31c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01w1-3613162940-0d7959f58923d7720b7a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0h00-4901120200-e21f1f6fe4c02cbce38e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0102000229-4248c6f33d014f47a24e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-07vu-7593030251-0e3ef15d1857aa17328d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0k9f-3239030000-b162809b348dc7344a95 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a6r-1500090704-08ef32e5a25ba27bb30b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004r-3900050301-217a4cdf524f05b46152 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03dl-3910022000-d04830789a6dacb7c7d7 | View in MoNA |
|---|
|
|---|