| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:23:56 UTC |
|---|
| Update Date | 2020-05-11 20:42:38 UTC |
|---|
| BMDB ID | BMDB0011745 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | N-Acetyl-L-methionine |
|---|
| Description | N-Acetyl-L-methionine, also known as L-(N-acetyl)methionine or methionamine, belongs to the class of organic compounds known as methionine and derivatives. Methionine and derivatives are compounds containing methionine or a derivative thereof resulting from reaction of methionine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. N-Acetyl-L-methionine is an extremely weak basic (essentially neutral) compound (based on its pKa). N-Acetyl-L-methionine exists in all living organisms, ranging from bacteria to humans. |
|---|
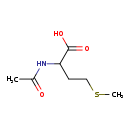
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Acetyl-L-methionine | HMDB | | Acetylmethionin | HMDB | | Acetylmethionine | HMDB | | DL-N-Acetylmethionine | HMDB | | L-(N-Acetyl)methionine | HMDB | | L-N-Acetyl-methionine | HMDB | | Methionamine | HMDB | | Methionin | HMDB | | N-Acetyl(methyl)homocysteine | HMDB | | N-Acetyl-methionine | HMDB | | N-Acetyl-S-methylhomocysteine | HMDB | | N-Acetylmethionine | HMDB | | Thiomedon | HMDB | | N-Acetylmethionine monopotassium salt | HMDB | | N-Acetylmethionine monosodium salt | HMDB | | N-Acetylmethionine, (DL)-isomer | HMDB | | N-Ac-L-methionine | HMDB | | Hepsan | HMDB | | N-Acetylmethionine, (D)-isomer | HMDB | | 2-[(1-Hydroxyethylidene)amino]-4-(methylsulfanyl)butanoate | Generator | | 2-[(1-Hydroxyethylidene)amino]-4-(methylsulphanyl)butanoate | Generator | | 2-[(1-Hydroxyethylidene)amino]-4-(methylsulphanyl)butanoic acid | Generator |
|
|---|
| Chemical Formula | C7H13NO3S |
|---|
| Average Molecular Weight | 191.248 |
|---|
| Monoisotopic Molecular Weight | 191.061613977 |
|---|
| IUPAC Name | 2-acetamido-4-(methylsulfanyl)butanoic acid |
|---|
| Traditional Name | L-methionine, N-acetyl- |
|---|
| CAS Registry Number | 65-82-7 |
|---|
| SMILES | CSCCC(NC(C)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C7H13NO3S/c1-5(9)8-6(7(10)11)3-4-12-2/h6H,3-4H2,1-2H3,(H,8,9)(H,10,11) |
|---|
| InChI Key | XUYPXLNMDZIRQH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as methionine and derivatives. Methionine and derivatives are compounds containing methionine or a derivative thereof resulting from reaction of methionine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Methionine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Methionine or derivatives
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Thia fatty acid
- Fatty acid
- Fatty acyl
- Acetamide
- Carboxamide group
- Secondary carboxylic acid amide
- Dialkylthioether
- Sulfenyl compound
- Thioether
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Organic oxide
- Organopnictogen compound
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Organic oxygen compound
- Hydrocarbon derivative
- Organic nitrogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 105.5 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 307mg/mL at 25 °C | Not Available | | LogP | -0.03 | MEYLAN,WM & HOWARD,PH (1995) |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-0v4j-2920000000-21fd3a4d7b12ac2aca3c | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 TMS) | splash10-0002-6900000000-3e9fc6c1775a4e3d390a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9300000000-c255d0240b6e3f4a5643 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-006w-9310000000-580909b614389c497c25 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-006x-0900000000-e657a0db51c30ae3be19 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0udl-1900000000-f56fad3e53b5ea9b2ecb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0002-9300000000-8202aca0e38266373acb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , negative | splash10-0005-1900000000-e28b73dfa967365c0250 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0f6x-3900000000-12868e3ab2754a2f9ee3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0900000000-137674c500bfd008d801 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udj-2900000000-8b99d2bc00227110da81 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-9300000000-d8b36ea8efa070adb63e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0005-4900000000-f1e7e9d2f55dc3f8e191 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-9300000000-bc39cc7c889e3f0d4390 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9000000000-012406ad4998287d85a8 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 500 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|