| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:32:52 UTC |
|---|
| Update Date | 2020-04-22 15:48:37 UTC |
|---|
| BMDB ID | BMDB0012128 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
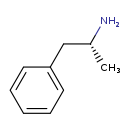
| Common Name | (R)-Amphetamine |
|---|
| Description | (R)-Amphetamine, also known as levamphetamine or centramina, belongs to the class of organic compounds known as amphetamines and derivatives. These are organic compounds containing or derived from 1-phenylpropan-2-amine. Based on a literature review a small amount of articles have been published on (R)-Amphetamine. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-Amphetamine | ChEBI | | (-)-Phenylisopropylamine | ChEBI | | (R)-alpha-Methylbenzeneethanamine | ChEBI | | (R)-alpha-Methylphenethylamine | ChEBI | | Levamfetamine | ChEBI | | Levamphetamine | ChEBI | | (R)-a-Methylbenzeneethanamine | Generator | | (R)-Α-methylbenzeneethanamine | Generator | | (R)-a-Methylphenethylamine | Generator | | (R)-Α-methylphenethylamine | Generator | | Amfetamine | MeSH | | Amphetamine | MeSH | | Amphetamine sulfate | MeSH | | Amphetamine sulfate (2:1) | MeSH | | Centramina | MeSH | | Desoxynorephedrin | MeSH | | Fenamine | MeSH | | Levoamphetamine | MeSH | | Mydrial | MeSH | | Phenamine | MeSH | | Phenopromin | MeSH | | Sulfate, amphetamine | MeSH | | Thyramine | MeSH | | L Amphetamine | MeSH | | Levo amphetamine | MeSH | | Levo-amphetamine | MeSH | | (R)-a-Methyl-benzeneethanamine | HMDB | | (R)-alpha-Methyl-benzeneethanamine | HMDB | | (R)-Phenaminum | HMDB | | (R)-Phenylisopropylamine | HMDB | | L-(R)-Amphetamine | HMDB | | L-a-Methylphenethylamine | HMDB | | L-alpha-Methylphenethylamine | HMDB | | L-Amphetamine | HMDB | | Miquel brand OF amfetamine sulfate | MeSH, HMDB |
|
|---|
| Chemical Formula | C9H13N |
|---|
| Average Molecular Weight | 135.2062 |
|---|
| Monoisotopic Molecular Weight | 135.104799421 |
|---|
| IUPAC Name | (2R)-1-phenylpropan-2-amine |
|---|
| Traditional Name | (-)-amphetamine |
|---|
| CAS Registry Number | 156-34-3 |
|---|
| SMILES | C[C@@H](N)CC1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C9H13N/c1-8(10)7-9-5-3-2-4-6-9/h2-6,8H,7,10H2,1H3/t8-/m1/s1 |
|---|
| InChI Key | KWTSXDURSIMDCE-MRVPVSSYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as amphetamines and derivatives. These are organic compounds containing or derived from 1-phenylpropan-2-amine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenethylamines |
|---|
| Direct Parent | Amphetamines and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Amphetamine or derivatives
- Phenylpropane
- Aralkylamine
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Primary amine
- Organonitrogen compound
- Primary aliphatic amine
- Amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9100000000-41224b447ebed58b4d86 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0900000000-b3a524d7c495f48a74c3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014r-1900000000-081ce3a25bbacda4ab98 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9300000000-5195de4351c8a02eb232 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-22cd79bae5466b52ae0a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-1900000000-a4e4f7fb040e60f9e5ba | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-4900000000-e652b091af97ea288b4c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kf-6900000000-4f5d22828acdcb13d83e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9000000000-c5766ace6b2031302336 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-c8535cbb285cac999590 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-9200000000-0f66be3239e5e65d6028 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9100000000-6720c563d36a88e31cd6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002f-9000000000-f91f5b5177c2d32668fd | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|