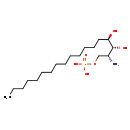| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:35:28 UTC |
|---|
| Update Date | 2020-04-22 15:49:27 UTC |
|---|
| BMDB ID | BMDB0012280 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Phytosphingosine-1-P |
|---|
| Description | Phytosphingosine-1-P, also known as PHS-1-phosphate, belongs to the class of organic compounds known as phosphosphingolipids. These are sphingolipids with a structure based on a sphingoid base that is attached to a phosphate head group. They differ from phosphonospingolipids which have a phosphonate head group. Phytosphingosine-1-P is a very strong basic compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| PHS-1-Phosphate | HMDB | | {[(2R,3R,4R)-2-amino-3,4-dihydroxyoctadecyl]oxy}phosphonate | Generator |
|
|---|
| Chemical Formula | C18H40NO6P |
|---|
| Average Molecular Weight | 397.4871 |
|---|
| Monoisotopic Molecular Weight | 397.259324529 |
|---|
| IUPAC Name | {[(2R,3R,4R)-2-amino-3,4-dihydroxyoctadecyl]oxy}phosphonic acid |
|---|
| Traditional Name | [(2R,3R,4R)-2-amino-3,4-dihydroxyoctadecyl]oxyphosphonic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCCCCCCCCCCCCC[C@@H](O)[C@H](O)[C@H](N)COP(O)(O)=O |
|---|
| InChI Identifier | InChI=1S/C18H40NO6P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-17(20)18(21)16(19)15-25-26(22,23)24/h16-18,20-21H,2-15,19H2,1H3,(H2,22,23,24)/t16-,17-,18-/m1/s1 |
|---|
| InChI Key | AYGOSKULTISFCW-KZNAEPCWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phosphosphingolipids. These are sphingolipids with a structure based on a sphingoid base that is attached to a phosphate head group. They differ from phosphonospingolipids which have a phosphonate head group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Sphingolipids |
|---|
| Sub Class | Phosphosphingolipids |
|---|
| Direct Parent | Phosphosphingolipids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sphingoid-1-phosphate or derivatives
- Phosphoethanolamine
- Monoalkyl phosphate
- Alkyl phosphate
- Organic phosphoric acid derivative
- Phosphoric acid ester
- 1,3-aminoalcohol
- 1,2-diol
- 1,2-aminoalcohol
- Secondary alcohol
- Organopnictogen compound
- Organic nitrogen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Primary aliphatic amine
- Amine
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Endosome
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01x0-2092000000-acc2f44cfa57dcd01b4c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-3009000000-8ebf59b673714024fe3b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-3009000000-dfaf95a262f32fa73ae1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-3009000000-b13c4300bd43cd6fe24d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udj-1109000000-ebc5dcf7ae08ad6f242e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f8c-6946000000-14b7651216a15069c22f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9100000000-793ba73f397ca7b12ee1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-1009000000-6a89f2941d4431cc984a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004j-9000000000-eed425d6e97a9cbe3c39 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-a5e502a2627af2048a1f | View in MoNA |
|---|
|
|---|