| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:39:35 UTC |
|---|
| Update Date | 2020-06-04 19:41:45 UTC |
|---|
| BMDB ID | BMDB0013124 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Propenoylcarnitine |
|---|
| Description | Propenoylcarnitine belongs to the class of organic compounds known as acyl carnitines. These are organic compounds containing a fatty acid with the carboxylic acid attached to carnitine through an ester bond. Thus, propenoylcarnitine is considered to be a fatty ester. Based on a literature review a significant number of articles have been published on Propenoylcarnitine. |
|---|
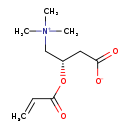
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3S)-3-(Prop-2-enoyloxy)-4-(trimethylazaniumyl)butanoate | ChEBI | | (3S)-3-(Prop-2-enoyloxy)-4-(trimethylazaniumyl)butanoic acid | Generator | | Propenoyl-L-carnitine | HMDB |
|
|---|
| Chemical Formula | C10H17NO4 |
|---|
| Average Molecular Weight | 215.2463 |
|---|
| Monoisotopic Molecular Weight | 215.115758037 |
|---|
| IUPAC Name | (3S)-3-(prop-2-enoyloxy)-4-(trimethylazaniumyl)butanoate |
|---|
| Traditional Name | (3S)-3-(prop-2-enoyloxy)-4-(trimethylammonio)butanoate |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | C[N+](C)(C)C[C@H](CC([O-])=O)OC(=O)C=C |
|---|
| InChI Identifier | InChI=1S/C10H17NO4/c1-5-10(14)15-8(6-9(12)13)7-11(2,3)4/h5,8H,1,6-7H2,2-4H3/t8-/m0/s1 |
|---|
| InChI Key | YUCNWOKTRWJLGY-QMMMGPOBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acyl carnitines. These are organic compounds containing a fatty acid with the carboxylic acid attached to carnitine through an ester bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acid esters |
|---|
| Direct Parent | Acyl carnitines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acyl-carnitine
- Dicarboxylic acid or derivatives
- Acrylic acid ester
- Quaternary ammonium salt
- Enoate ester
- Alpha,beta-unsaturated carboxylic ester
- Tetraalkylammonium salt
- Acrylic acid or derivatives
- Carboxylic acid salt
- Carboxylic acid ester
- Carboxylic acid
- Carboxylic acid derivative
- Organic salt
- Amine
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organopnictogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05fr-9000000000-75fc08c3d27feb518327 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-066v-2930000000-454a580179166d0507bb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052f-3900000000-b50c527fe3c21e151579 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pi0-9400000000-4054ae16ebec41595115 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-4190000000-363b169cd979e9082c96 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0nt9-6940000000-be8367f8d194c1c16d97 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uk9-9000000000-81ec5a1bdf939e2a5454 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0090000000-d39d73fb6e9c04d728f3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kr-9050000000-40bf40a4e27ee0de0686 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-9000000000-e9262cbaff8cb4ad0ba6 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| |
| Blood | Detected and Quantified | 0.029 +/- 0.004 uM | Not Specified | Not Specified | Normal | | details | | Liver | Detected and Quantified | 0.2 +/- 0.1 nmol/g of tissue | Not Specified | Not Specified | Normal | | details | | Longissimus Thoracis Muscle | Detected and Quantified | 1.8 +/- 0.2 nmol/g of tissue | Not Specified | Not Specified | Normal | | details | | Ruminal Fluid | Detected and Quantified | 0.03 +/- 0.01 uM | Not Specified | Not Specified | Normal | | details | | Semimembranosus Muscle | Detected and Quantified | 1.8 +/- 0.2 nmol/g of tissue | Not Specified | Not Specified | Normal | | details | | Testis | Detected and Quantified | 0.77 +/- 0.13 nmol/g of tissue | Not Specified | Not Specified | Normal | | details |
|
|---|