| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:40:07 UTC |
|---|
| Update Date | 2020-06-04 19:26:38 UTC |
|---|
| BMDB ID | BMDB0013429 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | PC(o-18:1(9Z)/18:2(9Z,12Z)) |
|---|
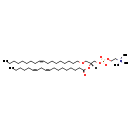
| Description | PC(O-18:1(9Z)/18:2(9Z,12Z)), also known as gpcho(18:1/18:2) or gpcho(36:3), belongs to the class of organic compounds known as 1-alkyl,2-acylglycero-3-phosphocholines. These are glycerophosphocholines that carry exactly one acyl chain attached to the glycerol moiety through an ester linkage at the O2-position, and one alkyl chain attached through an ether linkage at the O1-position. Thus, PC(O-18:1(9Z)/18:2(9Z,12Z)) is considered to be a glycerophosphocholine lipid molecule. PC(O-18:1(9Z)/18:2(9Z,12Z)) is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Oleyl-2-linoleoyl-sn-glycero-3-phosphocholine | HMDB | | Gpcho(18:1/18:2) | HMDB | | Gpcho(18:1n9/18:2n6) | HMDB | | Gpcho(18:1W9/18:2W6) | HMDB | | Gpcho(36:3) | HMDB | | Lecithin | HMDB | | PC Ae C36:3 | HMDB | | PC(18:1/18:2) | HMDB | | PC(18:1n9/18:2n6) | HMDB | | PC(18:1W9/18:2W6) | HMDB | | PC(36:3) | HMDB | | PC(O-36:3) | HMDB | | Phosphatidylcholine(18:1/18:2) | HMDB | | Phosphatidylcholine(18:1n9/18:2n6) | HMDB | | Phosphatidylcholine(18:1W9/18:2W6) | HMDB | | Phosphatidylcholine(36:3) | HMDB | | 1-(9Z-Octadecenyl)-2-(9Z,12Z-octadecadienoyl)-sn-glycero-3-phosphocholine | HMDB | | PC(o-18:1(9Z)/18:2(9Z,12Z)) | Lipid Annotator |
|
|---|
| Chemical Formula | C44H84NO7P |
|---|
| Average Molecular Weight | 770.114 |
|---|
| Monoisotopic Molecular Weight | 769.598540559 |
|---|
| IUPAC Name | trimethyl(2-{[(2R)-3-[(9Z)-octadec-9-en-1-yloxy]-2-[(9Z,12Z)-octadeca-9,12-dienoyloxy]propyl phosphonato]oxy}ethyl)azanium |
|---|
| Traditional Name | trimethyl(2-{[(2R)-3-[(9Z)-octadec-9-en-1-yloxy]-2-[(9Z,12Z)-octadeca-9,12-dienoyloxy]propyl phosphonato]oxy}ethyl)azanium |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCCCCCCC\C=C/CCCCCCCCOC[C@]([H])(COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCC\C=C/C\C=C/CCCCC |
|---|
| InChI Identifier | InChI=1S/C44H84NO7P/c1-6-8-10-12-14-16-18-20-22-24-26-28-30-32-34-36-39-49-41-43(42-51-53(47,48)50-40-38-45(3,4)5)52-44(46)37-35-33-31-29-27-25-23-21-19-17-15-13-11-9-7-2/h15,17,20-23,43H,6-14,16,18-19,24-42H2,1-5H3/b17-15-,22-20-,23-21-/t43-/m1/s1 |
|---|
| InChI Key | MFZLAZYUXHHSIA-HVODBARTSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1-alkyl,2-acylglycero-3-phosphocholines. These are glycerophosphocholines that carry exactly one acyl chain attached to the glycerol moiety through an ester linkage at the O2-position, and one alkyl chain attached through an ether linkage at the O1-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphocholines |
|---|
| Direct Parent | 1-alkyl,2-acylglycero-3-phosphocholines |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-alkyl,2-acylglycero-3-phosphocholine
- Phosphocholine
- Fatty acid ester
- Dialkyl phosphate
- Glycerol ether
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Fatty acyl
- Alkyl phosphate
- Tetraalkylammonium salt
- Quaternary ammonium salt
- Carboxylic acid ester
- Carboxylic acid derivative
- Dialkyl ether
- Ether
- Monocarboxylic acid or derivatives
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Amine
- Organic nitrogen compound
- Organooxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organic salt
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Intracellular membrane
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-9131231300-a6ab993d523240177de8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udr-8494143100-e3464cf31d3ef5f65d16 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f79-9064032000-6f4e2c960d045901b1d2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0070003900-cfd8f48252b0f668c197 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-016r-1090206300-0fca15fc8b31f6f3ba42 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-3090100000-57827747b948b140c268 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000000900-f8446695140eef013859 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0000000900-f8446695140eef013859 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0089-1900160700-9def314e2904596f2b55 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000000900-a77b4361de0929ecf0e7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-02t9-0050010900-8aba133c7dac02615311 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-4090400000-11515858e05c6a7d7ab7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000000090-3d6811548a7d3de72b59 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0000000090-3d6811548a7d3de72b59 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002f-0070301910-7f0aab79420610ffd4b2 | View in MoNA |
|---|
|
|---|