| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-06-25 21:50:23 UTC |
|---|
| Update Date | 2020-03-13 17:33:36 UTC |
|---|
| BMDB ID | BMDB0062096 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | beta-D-Fructofuranosyl-(2,1)-beta-D-Fructofuranose |
|---|
| Description | beta-D-Fructofuranosyl-(2,1)-beta-D-Fructofuranose belongs to the class of organic compounds known as c-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a C-glycosidic bond. Based on a literature review a significant number of articles have been published on beta-D-Fructofuranosyl-(2,1)-beta-D-Fructofuranose. |
|---|
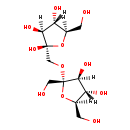
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| b-D-Fructofuranosyl-(2,1)-b-D-fructofuranose | Generator | | Β-D-fructofuranosyl-(2,1)-β-D-fructofuranose | Generator |
|
|---|
| Chemical Formula | C12H22O11 |
|---|
| Average Molecular Weight | 342.2965 |
|---|
| Monoisotopic Molecular Weight | 342.116211546 |
|---|
| IUPAC Name | (2R,3S,4S,5R)-2-({[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}methyl)-5-(hydroxymethyl)oxolane-2,3,4-triol |
|---|
| Traditional Name | (2R,3S,4S,5R)-2-({[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}methyl)-5-(hydroxymethyl)oxolane-2,3,4-triol |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@]1(O)[C@@]([H])(CO)O[C@](O)(CO[C@@]2(CO)O[C@]([H])(CO)[C@@]([H])(O)[C@]2([H])O)[C@@]1([H])O |
|---|
| InChI Identifier | InChI=1S/C12H22O11/c13-1-5-7(16)9(18)11(20,22-5)4-21-12(3-15)10(19)8(17)6(2-14)23-12/h5-10,13-20H,1-4H2/t5-,6-,7-,8-,9+,10+,11-,12+/m1/s1 |
|---|
| InChI Key | WOHYVFWWTVNXTP-IYDDCBTQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as c-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a C-glycosidic bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | C-glycosyl compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Disaccharide
- C-glycosyl compound
- Ketal
- Oxolane
- Secondary alcohol
- Hemiacetal
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Acetal
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 8 TMS) | splash10-0gb9-1960000000-8d0458f2338308846ae2 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 8 TMS) | splash10-0gb9-1961000000-0cfd2f317d147c29d052 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01qc-2904000000-10b28fb6d22218ff39f8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-1903000000-b70adf3ffd287ec37c56 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9300000000-a492a2428fca2ea6c72f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03fu-4904000000-4a9eb9173734034d8d67 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03fs-0900000000-78bf5aec0d5de766ca5c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0005-8900000000-4ed913acd03e8ef20136 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056u-0009000000-76c8529f540f4de9b8da | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000j-9613000000-56efa4c9b20e1f3a06a6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052v-9110000000-2f08422241ed33e6d0a1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01ox-0956000000-4fe547a3d4f722bd49e3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9643000000-84cb2d680aab4c9bcbbf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-9620000000-1c49981f1a021c6fac51 | View in MoNA |
|---|
|
|---|