| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-06-25 21:52:01 UTC |
|---|
| Update Date | 2020-05-05 18:40:39 UTC |
|---|
| BMDB ID | BMDB0062118 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Loganin |
|---|
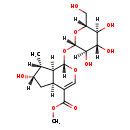
| Description | Loganin, also known as loganoside or 7-deoxyloganin, belongs to the class of organic compounds known as iridoid o-glycosides. These are iridoid monoterpenes containing a glycosyl (usually a pyranosyl) moiety linked to the iridoid skeleton. Thus, loganin is considered to be an isoprenoid lipid molecule. Loganin is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(beta-D-Glucopyranosyloxy)-1,4a,5,6,7,7a-hexahydro-6-hydroxy-7-methylcyclopenta[c]pyran-4-carboxylic acid methyl ester | ChEBI | | Loganoside | ChEBI | | 1-(b-D-Glucopyranosyloxy)-1,4a,5,6,7,7a-hexahydro-6-hydroxy-7-methylcyclopenta[c]pyran-4-carboxylate methyl ester | Generator | | 1-(b-D-Glucopyranosyloxy)-1,4a,5,6,7,7a-hexahydro-6-hydroxy-7-methylcyclopenta[c]pyran-4-carboxylic acid methyl ester | Generator | | 1-(beta-D-Glucopyranosyloxy)-1,4a,5,6,7,7a-hexahydro-6-hydroxy-7-methylcyclopenta[c]pyran-4-carboxylate methyl ester | Generator | | 1-(Β-D-glucopyranosyloxy)-1,4a,5,6,7,7a-hexahydro-6-hydroxy-7-methylcyclopenta[c]pyran-4-carboxylate methyl ester | Generator | | 1-(Β-D-glucopyranosyloxy)-1,4a,5,6,7,7a-hexahydro-6-hydroxy-7-methylcyclopenta[c]pyran-4-carboxylic acid methyl ester | Generator | | 7-Deoxyloganin | MeSH |
|
|---|
| Chemical Formula | C17H26O10 |
|---|
| Average Molecular Weight | 390.3823 |
|---|
| Monoisotopic Molecular Weight | 390.152597052 |
|---|
| IUPAC Name | methyl (1S,4aS,6S,7R,7aS)-6-hydroxy-7-methyl-1-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-1H,4aH,5H,6H,7H,7aH-cyclopenta[c]pyran-4-carboxylate |
|---|
| Traditional Name | loganin |
|---|
| CAS Registry Number | 18524-94-2 |
|---|
| SMILES | [H][C@]1(O)C[C@]2([H])C(=CO[C@@]([H])(O[C@]3([H])O[C@]([H])(CO)[C@@]([H])(O)[C@]([H])(O)[C@@]3([H])O)[C@]2([H])[C@@]1([H])C)C(=O)OC |
|---|
| InChI Identifier | InChI=1S/C17H26O10/c1-6-9(19)3-7-8(15(23)24-2)5-25-16(11(6)7)27-17-14(22)13(21)12(20)10(4-18)26-17/h5-7,9-14,16-22H,3-4H2,1-2H3/t6-,7+,9-,10+,11+,12+,13-,14+,16-,17-/m0/s1 |
|---|
| InChI Key | AMBQHHVBBHTQBF-UOUCRYGSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as iridoid o-glycosides. These are iridoid monoterpenes containing a glycosyl (usually a pyranosyl) moiety linked to the iridoid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Terpene glycosides |
|---|
| Direct Parent | Iridoid O-glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Iridoid o-glycoside
- Hexose monosaccharide
- Glycosyl compound
- Iridoid-skeleton
- O-glycosyl compound
- Bicyclic monoterpenoid
- Monoterpenoid
- Monosaccharide
- Oxane
- Cyclic alcohol
- Vinylogous ester
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Methyl ester
- Carboxylic acid ester
- Secondary alcohol
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Oxacycle
- Acetal
- Carboxylic acid derivative
- Polyol
- Organooxygen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Primary alcohol
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | |
|---|