| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-06-29 22:18:52 UTC |
|---|
| Update Date | 2020-06-04 20:41:37 UTC |
|---|
| BMDB ID | BMDB0062207 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Menatetrenone |
|---|
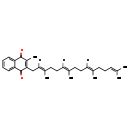
| Description | Menatetrenone, also known as menaquinone 4 or vitamin K2(20), belongs to the class of organic compounds known as menaquinones. These are vitamin K2 compounds consisting of a naphtho-1,4-quinone ring system, which is substituted at the 2-position by an isoprenyl side-chain, and usually, at the 3-position by a methyl group. Based on a literature review a significant number of articles have been published on Menatetrenone. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Methyl-3-(3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl)-1,4-naphthoquinone | ChEBI | | 2-Methyl-3-geranylgeranyl-1,4-naphthoquinone | ChEBI | | 2-Methyl-3-trans-tetraprenyl-1,4-naphthoquinone | ChEBI | | Menaquinone 4 | ChEBI | | Menaquinone K4 | ChEBI | | Menatetrenona | ChEBI | | Menatetrenonum | ChEBI | | MK-4 | ChEBI | | MK4 | ChEBI | | Vitamin K2(20) | ChEBI | | Vitamin MK 4 | ChEBI | | Vitamin K2 | Kegg | | Menaquinone-4 | ChEBI | | (e,e,e)-Isomer OF menatetrenone | MeSH, HMDB | | 2-Methyl-3-all-trans-tetraprenyl-1,4-naphthoquinone | MeSH, HMDB | | Kefton-2 | MeSH, HMDB | | Vitamin MK-4 | MeSH, HMDB | | Vitamin K 2 | MeSH | | Vitamin K quinone | MeSH | | Menaquinone | MeSH | | Menaquinones | MeSH |
|
|---|
| Chemical Formula | C31H40O2 |
|---|
| Average Molecular Weight | 444.659 |
|---|
| Monoisotopic Molecular Weight | 444.302830528 |
|---|
| IUPAC Name | 2-methyl-3-[(2E,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraen-1-yl]-1,4-dihydronaphthalene-1,4-dione |
|---|
| Traditional Name | menatetrenone |
|---|
| CAS Registry Number | 863-61-6 |
|---|
| SMILES | [H]\C(CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC1=C(C)C(=O)C2=CC=CC=C2C1=O)=C(\C)CCC=C(C)C |
|---|
| InChI Identifier | InChI=1S/C31H40O2/c1-22(2)12-9-13-23(3)14-10-15-24(4)16-11-17-25(5)20-21-27-26(6)30(32)28-18-7-8-19-29(28)31(27)33/h7-8,12,14,16,18-20H,9-11,13,15,17,21H2,1-6H3/b23-14+,24-16+,25-20+ |
|---|
| InChI Key | DKHGMERMDICWDU-GHDNBGIDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as menaquinones. These are vitamin K2 compounds consisting of a naphtho-1,4-quinone ring system, which is substituted at the 2-position by an isoprenyl side-chain, and usually, at the 3-position by a methyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Quinone and hydroquinone lipids |
|---|
| Direct Parent | Menaquinones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Menaquinone
- Diterpenoid
- Naphthoquinone
- Naphthalene
- Aryl ketone
- Quinone
- Benzenoid
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
- Mitochondria
|
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-014l-9430000000-936e4020e35c79ab80a8 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-014l-9430000000-936e4020e35c79ab80a8 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-003r-7891050000-fb45abd0445fd13b3287 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0000900000-a5b1daed1e13ae9cdc49 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0101900000-63826c82750026b58641 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00fr-2925400000-e48e5e86379a4048f95e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0000900000-87177730a5d4e2d424bb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000f-0902700000-70d2be403d22d9bb2275 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002s-2923100000-7822153ffca689168909 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0221900000-da2fa06091b3f02f7f62 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0550-0968100000-d9351f8233d2638c6648 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9673000000-f5a80c46af487a0ae786 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-2137900000-9bfb9ad4ac1145915e57 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0019-3419100000-17e116bda37eb38893dc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-007a-3901000000-9c3d341cbd4ad0feec93 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|