| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-07-17 17:43:37 UTC |
|---|
| Update Date | 2020-05-21 16:27:24 UTC |
|---|
| BMDB ID | BMDB0062493 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | TG(14:1(9Z)/14:1(9Z)/16:1(9Z)) |
|---|
| Description | TG(14:1(9Z)/14:1(9Z)/16:1(9Z)), also known as tag(14:1/14:1/16:1) or tag(44:3), belongs to the class of organic compounds known as triacylglycerols. These are glycerides consisting of three fatty acid chains covalently bonded to a glycerol molecule through ester linkages. TG(14:1(9Z)/14:1(9Z)/16:1(9Z)) is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). TG(14:1(9Z)/14:1(9Z)/16:1(9Z)) can be biosynthesized from DG(14:1(9Z)/14:1(9Z)/0:0) and palmitoleyl-CoA; which is mediated by the enzyme diacylglycerol O-acyltransferase. In cattle, TG(14:1(9Z)/14:1(9Z)/16:1(9Z)) is involved in the metabolic pathway called de novo triacylglycerol biosynthesis TG(14:1(9Z)/14:1(9Z)/16:1(9Z)) pathway. |
|---|
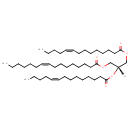
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(9Z-Tetradecenoyl)-2-(9Z-tetradecenoyl)-3-(9Z-hexadecenoyl)-glycerol | HMDB | | 1-Myristoleoyl-2-myristoleoyl-3-palmitoleoyl-glycerol | HMDB | | TAG(14:1/14:1/16:1) | HMDB | | TAG(44:3) | HMDB | | TG(14:1/14:1/16:1) | HMDB | | TG(44:3) | HMDB | | Tracylglycerol(14:1/14:1/16:1) | HMDB | | Tracylglycerol(44:3) | HMDB | | Triacylglycerol | HMDB | | Triglyceride | HMDB | | TG(14:1(9Z)/14:1(9Z)/16:1(9Z)) | Lipid Annotator |
|
|---|
| Chemical Formula | C47H84O6 |
|---|
| Average Molecular Weight | 745.183 |
|---|
| Monoisotopic Molecular Weight | 744.626790425 |
|---|
| IUPAC Name | (2S)-2,3-bis[(9Z)-tetradec-9-enoyloxy]propyl (9Z)-hexadec-9-enoate |
|---|
| Traditional Name | (2S)-2,3-bis[(9Z)-tetradec-9-enoyloxy]propyl (9Z)-hexadec-9-enoate |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@](COC(=O)CCCCCCC\C=C/CCCC)(COC(=O)CCCCCCC\C=C/CCCCCC)OC(=O)CCCCCCC\C=C/CCCC |
|---|
| InChI Identifier | InChI=1S/C47H84O6/c1-4-7-10-13-16-19-22-23-26-28-31-34-37-40-46(49)52-43-44(53-47(50)41-38-35-32-29-25-21-18-15-12-9-6-3)42-51-45(48)39-36-33-30-27-24-20-17-14-11-8-5-2/h14-15,17-19,22,44H,4-13,16,20-21,23-43H2,1-3H3/b17-14-,18-15-,22-19-/t44-/m0/s1 |
|---|
| InChI Key | SVKNSUKRPRJLMQ-QSDZTSERSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as triacylglycerols. These are glycerides consisting of three fatty acid chains covalently bonded to a glycerol molecule through ester linkages. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerolipids |
|---|
| Sub Class | Triradylcglycerols |
|---|
| Direct Parent | Triacylglycerols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triacyl-sn-glycerol
- Tricarboxylic acid or derivatives
- Fatty acid ester
- Fatty acyl
- Carboxylic acid ester
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Adiposome
- Cell membrane
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 16.06 | Extrapolated |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000000900-2835fc4f5ac956239126 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0000000900-2835fc4f5ac956239126 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014p-0000990700-e3daa71087f51f4ffba7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0kbu-0090010100-f1f22fb71201bef3d949 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0kdr-0090000000-6cf20212d30a5c4852d3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0kdi-1090000000-e43310c0a30ed3a94c58 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000000900-9d26a2a0d2a0313e7e17 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0000000900-9d26a2a0d2a0313e7e17 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014p-0030990700-632b81d787d5e0837809 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-2210246900-b16dcbfe3a05d55f49e6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-5050129200-c7e1ce4704dc5b5e591e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-2690121000-31fc0de1a1d31993d154 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000000900-8d746585b31839d582c6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0000000900-8d746585b31839d582c6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0y4i-0090090900-3ebec39175bbf77b84db | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00kf-0090040600-e2182e6c80083986d522 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0uxr-0090000000-a85ac9af3a12e29b4d48 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0zi0-1090000000-2d4f214aaa80f885492c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000000900-27c644004cb6bcf16a85 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0000000900-27c644004cb6bcf16a85 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0000000900-27c644004cb6bcf16a85 | View in MoNA |
|---|
|
|---|