| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-07-17 17:47:45 UTC |
|---|
| Update Date | 2020-03-13 17:36:40 UTC |
|---|
| BMDB ID | BMDB0062542 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
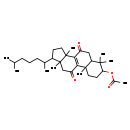
| Common Name | 7,11-Dioxolanost-8-en-3-yl acetate |
|---|
| Description | 7,11-Dioxolanost-8-en-3-yl acetate belongs to the class of organic compounds known as triterpenoids. These are terpene molecules containing six isoprene units. Based on a literature review a significant number of articles have been published on 7,11-Dioxolanost-8-en-3-yl acetate. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 7,11-Dioxolanost-8-en-3-yl acetic acid | Generator | | 2,6,6,11,15-Pentamethyl-14-(6-methylheptan-2-yl)-9,17-dioxotetracyclo[8.7.0.0,.0,]heptadec-1(10)-en-5-yl acetic acid | HMDB |
|
|---|
| Chemical Formula | C32H50O4 |
|---|
| Average Molecular Weight | 498.748 |
|---|
| Monoisotopic Molecular Weight | 498.37091009 |
|---|
| IUPAC Name | 2,6,6,11,15-pentamethyl-14-(6-methylheptan-2-yl)-9,17-dioxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-1(10)-en-5-yl acetate |
|---|
| Traditional Name | 2,6,6,11,15-pentamethyl-14-(6-methylheptan-2-yl)-9,17-dioxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-1(10)-en-5-yl acetate |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CC(C)CCCC(C)C1CCC2(C)C3=C(C(=O)CC12C)C1(C)CCC(OC(C)=O)C(C)(C)C1CC3=O |
|---|
| InChI Identifier | InChI=1S/C32H50O4/c1-19(2)11-10-12-20(3)22-13-16-31(8)28-23(34)17-25-29(5,6)26(36-21(4)33)14-15-30(25,7)27(28)24(35)18-32(22,31)9/h19-20,22,25-26H,10-18H2,1-9H3 |
|---|
| InChI Key | MMVITYGCRCBTDU-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as triterpenoids. These are terpene molecules containing six isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Triterpenoids |
|---|
| Direct Parent | Triterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triterpenoid
- Steroid ester
- 11-oxosteroid
- Oxosteroid
- 7-oxosteroid
- Steroid
- Cyclohexenone
- Ketone
- Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Organooxygen compound
- Hydrocarbon derivative
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052b-0000900000-2302e938b7389ef80765 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ads-1002900000-6715a9645d22f90925b0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ba-8342900000-4e8122b62b4d303947b4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-052b-0000900000-7cedd3a59763e93b237c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-2000900000-621fea1bae50d6bae64b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4u-5010900000-0bcd7e90e075864c7c0d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000b-9106800000-5e975fb5553e85c3d2a9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0aor-9003100000-1b71cf9715880473ed6d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9106000000-24b3764bca9cdde04a7c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-052b-8000900000-cda26d475bce243130bf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9000200000-5523ea838adec1aed592 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052b-4000900000-8e571999b69ea76f0d99 | View in MoNA |
|---|
|
|---|