| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-07-17 17:48:32 UTC |
|---|
| Update Date | 2020-03-13 17:36:45 UTC |
|---|
| BMDB ID | BMDB0062551 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
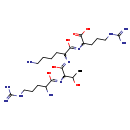
| Common Name | Arg-Thr-Lys-Arg |
|---|
| Description | Arg-Thr-Lys-Arg belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. Based on a literature review a small amount of articles have been published on Arg-Thr-Lys-Arg. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-{[6-amino-2-({2-[(2-amino-5-carbamimidamido-1-hydroxypentylidene)amino]-1,3-dihydroxybutylidene}amino)-1-hydroxyhexylidene]amino}-5-carbamimidamidopentanoate | HMDB |
|
|---|
| Chemical Formula | C22H45N11O6 |
|---|
| Average Molecular Weight | 559.673 |
|---|
| Monoisotopic Molecular Weight | 559.355428213 |
|---|
| IUPAC Name | 2-{[6-amino-2-({2-[(2-amino-5-carbamimidamido-1-hydroxypentylidene)amino]-1,3-dihydroxybutylidene}amino)-1-hydroxyhexylidene]amino}-5-carbamimidamidopentanoic acid |
|---|
| Traditional Name | 2-{[6-amino-2-({2-[(2-amino-5-carbamimidamido-1-hydroxypentylidene)amino]-1,3-dihydroxybutylidene}amino)-1-hydroxyhexylidene]amino}-5-carbamimidamidopentanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CC(O)C(N=C(O)C(N)CCCNC(N)=N)C(O)=NC(CCCCN)C(O)=NC(CCCNC(N)=N)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C22H45N11O6/c1-12(34)16(33-17(35)13(24)6-4-10-29-21(25)26)19(37)31-14(7-2-3-9-23)18(36)32-15(20(38)39)8-5-11-30-22(27)28/h12-16,34H,2-11,23-24H2,1H3,(H,31,37)(H,32,36)(H,33,35)(H,38,39)(H4,25,26,29)(H4,27,28,30) |
|---|
| InChI Key | UPLCTTWJNCYYAW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid amide
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- Fatty amide
- N-acyl-amine
- Fatty acyl
- Fatty acid
- Amino acid or derivatives
- Carboxamide group
- Guanidine
- Amino acid
- Secondary alcohol
- Secondary carboxylic acid amide
- Propargyl-type 1,3-dipolar organic compound
- Carboximidamide
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic 1,3-dipolar compound
- Primary aliphatic amine
- Organic nitrogen compound
- Organic oxide
- Amine
- Carbonyl group
- Alcohol
- Organooxygen compound
- Organopnictogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Primary amine
- Organonitrogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ffx-0402190000-055a1b2eb86922899452 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0200-3914220000-4d09f00283f28d3bb2b6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03fr-9812000000-7c83a5c8817819f211c8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0avm-1110790000-040e40350f28856bf3e1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0aba-4211940000-262201e7e6841a63120e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9211000000-04da63ef35a2e7f6e3b3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000090000-9f93a1d574b75cd8a634 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052e-1590150000-9cbd19743093d3ee7f00 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-6931000000-b60e30378d4a62e120c1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dl-0012090000-30eadc907fa3b3633a7c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01r6-2622190000-a946268b745a51e1a59b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0259-8900000000-d499e1cc8f506b57c257 | View in MoNA |
|---|
|
|---|