| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-07-17 17:48:37 UTC |
|---|
| Update Date | 2020-03-13 17:36:46 UTC |
|---|
| BMDB ID | BMDB0062552 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Asn-Arg-Ala-Ile |
|---|
| Description | Asn-arg-ala-ile belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. Asn-arg-ala-ile is possibly soluble (in water) and a very strong basic compound (based on its pKa). |
|---|
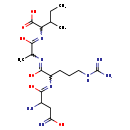
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-({2-[(2-{[2-amino-1-hydroxy-3-(C-hydroxycarbonimidoyl)propylidene]amino}-5-carbamimidamido-1-hydroxypentylidene)amino]-1-hydroxypropylidene}amino)-3-methylpentanoate | HMDB |
|
|---|
| Chemical Formula | C19H36N8O6 |
|---|
| Average Molecular Weight | 472.547 |
|---|
| Monoisotopic Molecular Weight | 472.275780911 |
|---|
| IUPAC Name | 2-({2-[(2-{[2-amino-1-hydroxy-3-(C-hydroxycarbonimidoyl)propylidene]amino}-5-carbamimidamido-1-hydroxypentylidene)amino]-1-hydroxypropylidene}amino)-3-methylpentanoic acid |
|---|
| Traditional Name | 2-({2-[(2-{[2-amino-1-hydroxy-3-(C-hydroxycarbonimidoyl)propylidene]amino}-5-carbamimidamido-1-hydroxypentylidene)amino]-1-hydroxypropylidene}amino)-3-methylpentanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCC(C)C(N=C(O)C(C)N=C(O)C(CCCNC(N)=N)N=C(O)C(N)CC(O)=N)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C19H36N8O6/c1-4-9(2)14(18(32)33)27-15(29)10(3)25-17(31)12(6-5-7-24-19(22)23)26-16(30)11(20)8-13(21)28/h9-12,14H,4-8,20H2,1-3H3,(H2,21,28)(H,25,31)(H,26,30)(H,27,29)(H,32,33)(H4,22,23,24) |
|---|
| InChI Key | SVSBLOSMLPLILN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- Asparagine or derivatives
- Isoleucine or derivatives
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- Alpha-amino acid amide
- Alanine or derivatives
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- Branched fatty acid
- Methyl-branched fatty acid
- Fatty acyl
- Fatty acid
- N-acyl-amine
- Fatty amide
- Amino acid
- Guanidine
- Secondary carboxylic acid amide
- Primary carboxylic acid amide
- Carboxamide group
- Amino acid or derivatives
- Carboxylic acid
- Carboximidamide
- Propargyl-type 1,3-dipolar organic compound
- Monocarboxylic acid or derivatives
- Organic 1,3-dipolar compound
- Organic nitrogen compound
- Primary aliphatic amine
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Amine
- Organopnictogen compound
- Organic oxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-4443900000-6558550a088c828735b7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01qi-9441100000-a6d0e4883434be1b28e4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01q3-9310000000-ada9f3a1574fb847f764 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0fi0-1011900000-90d3c38b7644ac441648 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0r03-8314900000-42dd184b97c9bae80553 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9410000000-9bf98bf3366505e8f63a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0001900000-8a3dc2450e084366997b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-1216900000-1b2287b484fe5f8062ec | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02t9-8900000000-aa098b12e4cbb705979e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0001900000-6db7580d9926f39b51c7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001l-3935500000-0aeb5fc4e7e95a1898f6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000x-5931000000-9e6db1c35a59a21eca1a | View in MoNA |
|---|
|
|---|