| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-07-17 17:50:06 UTC |
|---|
| Update Date | 2020-03-13 17:36:53 UTC |
|---|
| BMDB ID | BMDB0062566 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Lys-Asp-Tyr |
|---|
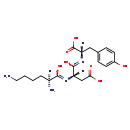
| Description | Lys-asp-tyr, also known as K-D-y or L-lys-L-asp-L-tyr, belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. Lys-asp-tyr is possibly soluble (in water) and a very strong basic compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| K-D-Y | ChEBI | | KDY | ChEBI | | L-Lys-L-asp-L-tyr | ChEBI |
|
|---|
| Chemical Formula | C19H28N4O7 |
|---|
| Average Molecular Weight | 424.454 |
|---|
| Monoisotopic Molecular Weight | 424.195799258 |
|---|
| IUPAC Name | (3S)-3-{[(1S)-1-carboxy-2-(4-hydroxyphenyl)ethyl]-C-hydroxycarbonimidoyl}-3-{[(2S)-2,6-diamino-1-hydroxyhexylidene]amino}propanoic acid |
|---|
| Traditional Name | (3S)-3-{[(1S)-1-carboxy-2-(4-hydroxyphenyl)ethyl]-C-hydroxycarbonimidoyl}-3-{[(2S)-2,6-diamino-1-hydroxyhexylidene]amino}propanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@](N)(CCCCN)C(O)=N[C@@]([H])(CC(O)=O)C(O)=N[C@@]([H])(CC1=CC=C(O)C=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C19H28N4O7/c20-8-2-1-3-13(21)17(27)22-14(10-16(25)26)18(28)23-15(19(29)30)9-11-4-6-12(24)7-5-11/h4-7,13-15,24H,1-3,8-10,20-21H2,(H,22,27)(H,23,28)(H,25,26)(H,29,30)/t13-,14-,15-/m0/s1 |
|---|
| InChI Key | NTBFKPBULZGXQL-KKUMJFAQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- Tyrosine or derivatives
- Phenylalanine or derivatives
- Aspartic acid or derivatives
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- N-acyl-l-alpha-amino acid
- Alpha-amino acid amide
- 3-phenylpropanoic-acid
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- Amphetamine or derivatives
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Fatty amide
- N-acyl-amine
- Benzenoid
- Fatty acyl
- Dicarboxylic acid or derivatives
- Monocyclic benzene moiety
- Amino acid or derivatives
- Amino acid
- Carboxamide group
- Secondary carboxylic acid amide
- Carboxylic acid
- Amine
- Hydrocarbon derivative
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Organic oxide
- Organic nitrogen compound
- Primary aliphatic amine
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0836900000-0efdbe54370f116e1ee6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-2922000000-bbd8c8a248d8ad227e0a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pc0-6900000000-d566da55608cbcbdb24c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-05i0-0226900000-3ae27990d631d68a0e36 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-06vi-0879400000-44416bd44fd85db0c55e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-005c-5910000000-864fcc8c2ac1542ed3bb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0201900000-f5e90b9b4bf39eba7fb0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-9401100000-4851e0dca35b3345ee90 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a59-9500000000-02b0a401287b4b41a521 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0100900000-cc9ad6d7dd8e77722e89 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03fr-2916000000-264d29f52d5e4abcb8bd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9610000000-ed52dd308a1c33d9b12f | View in MoNA |
|---|
|
|---|