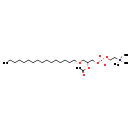
| (R)-7-(Acetyloxy)-4-hydroxy-N,N,N-trimethyl-3,5,9-trioxa-4-phosphapentacosan-1-aminium hydroxide inner salt 4-oxide | ChEBI |
| 1-Hexadecyl-2-acetyl-sn-glycero-3-phosphocholine | ChEBI |
| 1-O-Hexadecyl paf | ChEBI |
| 1-O-Hexadecyl-2-acetyl-sn-glycero-3-phosphocholine | ChEBI |
| 1-O-Hexadecyl-2-acetyl-sn-glyceryl-3-phosphorylcholine | ChEBI |
| 1-O-Hexadecyl-2-O-acetyl-sn-glycero-3-phosophocholine | ChEBI |
| 1-O-Hexadecyl-2-O-acetyl-sn-glyceryl-3-phosphorylcholine | ChEBI |
| 1-O-Hexadecyl-platelet-activating factor | ChEBI |
| C16 PAF | ChEBI |
| C16-PAF | ChEBI |
| C16:0 PAF | ChEBI |
| PAF | ChEBI |
| Platelet activating factor | ChEBI |
| Platelet-activating factor | ChEBI |
| Platelet-activating factor C16 | ChEBI |
| 1-Hexadecyl-2-acetyl-glycerophosphocholine | HMDB |
| APRL | HMDB |
| Hexadecyl-paf-acether | HMDB |
| 1-Hexadecyl-2-acetyl-glycero-3-phosphocholine | HMDB |
| HD-PAF | HMDB |
| PAF-16 CPD | HMDB |
| HAG-PC | HMDB |
| HAGPC | HMDB |
| Antihypertensive polar renomedullary lipid | HMDB |
| Aggregating factor, platelet | MeSH |
| PAF-acether | MeSH |
| Phosphorylcholine, acetyl glyceryl ether | MeSH |
| Platelet-activating substance | MeSH |
| AGEPC | MeSH |
| Platelet activating substance | MeSH |
| Platelet aggregation enhancing factor | MeSH |
| Factor, platelet activating | MeSH |
| PAF acether | MeSH |
| Thrombocyte aggregating activity | MeSH |
| 1 Alkyl 2 acetyl sn glycerophosphocholine | MeSH |
| 1-Alkyl-2-acetyl-sn-glycerophosphocholine | MeSH |
| Acetyl glyceryl ether phosphorylcholine | MeSH |
| Platelet aggregating factor | MeSH |
| 1-Hexadecyl-2-acetyl-GPC | HMDB |
| 1-Hexadecyl-2-acetyl-sn-glycero-phosphatidylcholine | HMDB |
| 1-O-Hexadecyl-2-O-acetyl-sn-glycero-3-phosphocholine | HMDB |
| 1-O-Hexadecyl-2-acetyl-sn-glycerophosphocholine | HMDB |
| Acetyl-glyceryl-ether-phosphorylcholine | HMDB |
| Blood platelet-activating factor | HMDB |
| Blood platelet-activating factor acether | HMDB |
| C16-PAF acether | HMDB |
| GPC(16:0/2:0) | HMDB |
| GPC(18:0) | HMDB |
| GPC(O-16:0/2:0) | HMDB |
| GPCho(16:0/2:0) | HMDB |
| GPCho(18:0) | HMDB |
| GPCho(O-16:0/2:0) | HMDB |
| PAF 16 | HMDB |
| PAF C 16 | HMDB |
| PC(16:0/2:0) | HMDB |
| PC(18:0) | HMDB |
| PC(O-16:0/2:0) | HMDB |
| Phosphatidylcholine(16:0/2:0) | HMDB |
| Phosphatidylcholine(18:0) | HMDB |
| Phosphatidylcholine(O-16:0/2:0) | HMDB |
| Platelet-activating factor (1-O-hexadecyl) | HMDB |
| Platelet-activating factor acether | HMDB |