| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-10-03 16:33:24 UTC |
|---|
| Update Date | 2020-06-04 18:57:38 UTC |
|---|
| BMDB ID | BMDB0063612 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
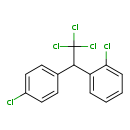
| Common Name | o,p′-DDT |
|---|
| Description | (-)-1-Chloro-2-(2,2,2-trichloro-1-(4-chlorophenyl)ethyl)benzene, also known as O,p'-DDT or 2-(2-chlorophenyl)-2-(4-chlorophenyl)-1,1,1-trichloroethane, belongs to the class of organic compounds known as diphenylmethanes. Diphenylmethanes are compounds containing a diphenylmethane moiety, which consists of a methane wherein two hydrogen atoms are replaced by two phenyl groups. Based on a literature review a small amount of articles have been published on (-)-1-Chloro-2-(2,2,2-trichloro-1-(4-chlorophenyl)ethyl)benzene. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| O,P'-DDT | Kegg | | 2-(2-Chlorophenyl)-2-(4-chlorophenyl)-1,1,1-trichloroethane | Kegg | | O,p-DDT | HMDB | | 1,1,1-Trichloro-2-(2-chlorophenyl)-2-(4-chlorophenyl)ethane | HMDB | | O,P'-dichlorodiphenyltrichloroethane | HMDB | | 2,4'-DDT | HMDB | | O,P'-DDT, (R)-isomer | HMDB |
|
|---|
| Chemical Formula | C14H9Cl5 |
|---|
| Average Molecular Weight | 354.486 |
|---|
| Monoisotopic Molecular Weight | 351.914688823 |
|---|
| IUPAC Name | 1-chloro-2-[2,2,2-trichloro-1-(4-chlorophenyl)ethyl]benzene |
|---|
| Traditional Name | 1-chloro-2-[2,2,2-trichloro-1-(4-chlorophenyl)ethyl]benzene |
|---|
| CAS Registry Number | 789-02-6 |
|---|
| SMILES | ClC1=CC=C(C=C1)C(C1=CC=CC=C1Cl)C(Cl)(Cl)Cl |
|---|
| InChI Identifier | InChI=1S/C14H9Cl5/c15-10-7-5-9(6-8-10)13(14(17,18)19)11-3-1-2-4-12(11)16/h1-8,13H |
|---|
| InChI Key | CVUGPAFCQJIYDT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as diphenylmethanes. Diphenylmethanes are compounds containing a diphenylmethane moiety, which consists of a methane wherein two hydrogen atoms are replaced by two phenyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Diphenylmethanes |
|---|
| Direct Parent | Diphenylmethanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diphenylmethane
- Halobenzene
- Chlorobenzene
- Aryl halide
- Aryl chloride
- Hydrocarbon derivative
- Organochloride
- Organohalogen compound
- Alkyl halide
- Alkyl chloride
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00kr-2392000000-a8a0544694e39d6ea0c1 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0009000000-b3d1428ff909538e6d29 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0009000000-858fddcfd4fab7a0c23e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0039000000-04ea21cd239ca4d3cbef | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-503c1f8788c6431fa214 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0009000000-189527111b16976360f7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-0019000000-e62775a4a03f14c4750d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0009000000-dd77118949b674041bd9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0009000000-dd77118949b674041bd9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uy3-0094000000-bca379e9ad2010d54630 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-17d9b4a570d431baa8a9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0009000000-17d9b4a570d431baa8a9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uxr-0649000000-3d4c624f57c92fd854a6 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-000i-1490000000-cbd1d026223f44bf4594 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 50.18 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|