| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-10-03 16:33:30 UTC |
|---|
| Update Date | 2020-06-04 19:11:09 UTC |
|---|
| BMDB ID | BMDB0063613 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Oxolinic Acid |
|---|
| Description | Oxolinic acid is a quinolinemonocarboxylic acid having the carboxy group at position 7 as well as oxo and ethyl groups at positions 4 and 1 respectively and a dioxolo ring fused at the 5- and 6-positions. A synthetic antibiotic, it is used in veterinary medicine for the treatment of bacterial infections in cattle, pigs and poultry. It has a role as an antiinfective agent, an antibacterial drug, an enzyme inhibitor, an antimicrobial agent and an antifungal agent. It is a quinolinemonocarboxylic acid, an organic heterotricyclic compound, an aromatic carboxylic acid, an oxacycle and a quinolone antibiotic. It is a conjugate acid of an oxolinate. |
|---|
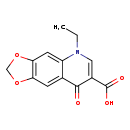
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Ethyl-1,4-dihydro-6,7-methylenedioxy-4-oxo-3-quinolinecarboxylic acid | ChEBI | | 1-Ethyl-6,7-methylenedioxy-4-quinolone-3-carboxylic acid | ChEBI | | 5-Ethyl-5,8-dihydro-8-oxo-1,3-dioxolo(4,5-g)quinoline-7-carboxylic acid | ChEBI | | Acide oxolinique | ChEBI | | Acido oxolinico | ChEBI | | Acidum oxolinicum | ChEBI | | OA | ChEBI | | Aqualinic | Kegg | | 1-Ethyl-1,4-dihydro-6,7-methylenedioxy-4-oxo-3-quinolinecarboxylate | Generator | | 1-Ethyl-6,7-methylenedioxy-4-quinolone-3-carboxylate | Generator | | 5-Ethyl-5,8-dihydro-8-oxo-1,3-dioxolo(4,5-g)quinoline-7-carboxylate | Generator | | Oxolinate | Generator | | Acid, oxolinic | MeSH | | Gramurin | MeSH | | Oxolinate, sodium | MeSH | | Sodium oxolinate | MeSH |
|
|---|
| Chemical Formula | C13H11NO5 |
|---|
| Average Molecular Weight | 261.2301 |
|---|
| Monoisotopic Molecular Weight | 261.063722467 |
|---|
| IUPAC Name | 5-ethyl-8-oxo-2H,5H,8H-[1,3]dioxolo[4,5-g]quinoline-7-carboxylic acid |
|---|
| Traditional Name | ossian |
|---|
| CAS Registry Number | 14698-29-4 |
|---|
| SMILES | CCN1C=C(C(O)=O)C(=O)C2=CC3=C(OCO3)C=C12 |
|---|
| InChI Identifier | InChI=1S/C13H11NO5/c1-2-14-5-8(13(16)17)12(15)7-3-10-11(4-9(7)14)19-6-18-10/h3-5H,2,6H2,1H3,(H,16,17) |
|---|
| InChI Key | KYGZCKSPAKDVKC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as quinoline carboxylic acids. These are quinolines in which the quinoline ring system is substituted by a carboxyl group at one or more positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Quinolines and derivatives |
|---|
| Sub Class | Quinoline carboxylic acids |
|---|
| Direct Parent | Quinoline carboxylic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Quinoline-3-carboxylic acid
- Dihydroquinolone
- Dihydroquinoline
- Benzodioxole
- Pyridine carboxylic acid
- Pyridine carboxylic acid or derivatives
- Pyridine
- Benzenoid
- Vinylogous amide
- Heteroaromatic compound
- Acetal
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Azacycle
- Oxacycle
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00l6-0490000000-237ab4e22ca8c87c87f9 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-03di-0090000000-42a1a31f158e9bef1a7d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-03di-0790000000-0196f4b3fe02d848ac04 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-03di-0920000000-8df3687ee0c2e91f987f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-03di-0090000000-24298432855bfc523660 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-03di-0090000000-0511184317d1b6d18d3f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-03di-0090000000-95faf056ba91e8007c9b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-03dl-0290000000-db6274d8128c051d14b8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-03e9-0960000000-c51deba0c41087d2cab2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-03e9-0910000000-bb9589ba074c27740963 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0ho0-4900000000-a825bf27d8d3c0aee71f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-02di-9500000000-bccf49f73f4aeba1dfa3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-004i-9100000000-55f0b933bb95b8ac1a52 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-03e9-0910000000-3c81458019c3bf0c9692 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-03e9-0960000000-f0f7c0d17b8af04334ce | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-03dl-0290000000-bf8142d91cf36f4709f4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-03di-0090000000-0511184317d1b6d18d3f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-03di-0090000000-9000f1dd05ac3d9ad631 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 50V, Positive | splash10-03di-0920000000-741ae3bcdfd5e8398bf6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-03di-0790000000-a21c32a280f959c2466b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0090000000-37dc156faa14512ceaab | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0296-0090000000-4381353df0b7e7aeee1d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0wtc-0790000000-e290c90d551c56e1ef77 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-02t9-0090000000-8aae635e5bdea056ef12 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0590000000-4a91cc6c672c3b18ada6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000l-0920000000-cd83ad14bff62acfdf66 | View in MoNA |
|---|
|
|---|
| General References | - Chen D, Yu J, Tao Y, Pan Y, Xie S, Huang L, Peng D, Wang X, Wang Y, Liu Z, Yuan Z: Qualitative screening of veterinary anti-microbial agents in tissues, milk, and eggs of food-producing animals using liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2016 Apr 1;1017-1018:82-88. doi: 10.1016/j.jchromb.2016.02.037. Epub 2016 Mar 3. [PubMed:26950031 ]
|
|---|