| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-10-03 16:34:04 UTC |
|---|
| Update Date | 2020-06-04 18:57:30 UTC |
|---|
| BMDB ID | BMDB0063619 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
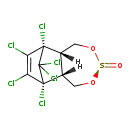
| Common Name | beta-endosulfan |
|---|
| Description | Endosulfan II is a polychlorinated compound used for controlling a variety of insects. It is practically water-insoluble, but readily adheres to clay particles and persists in soil and water for several years. Its mode of action involves repetitive nerve-discharges positively correlated to increase in temperature. This compound is extremely toxic to most fish (From Comp Biochem Physiol (C) 1993 Jul;105(3):347-61). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6-Hydroxy-1-methyl-1,2,3,4-tetrahydro-b-carboline | Generator | | 6-Hydroxy-1-methyl-1,2,3,4-tetrahydro-β-carboline | Generator | | b-endoSulfan | HMDB | | b-endoSulphan | HMDB | | beta-endoSulphan | HMDB | | Β-endosulfan | HMDB | | Β-endosulphan | HMDB |
|
|---|
| Chemical Formula | C9H6Cl6O3S |
|---|
| Average Molecular Weight | 406.9 |
|---|
| Monoisotopic Molecular Weight | 403.8168814 |
|---|
| IUPAC Name | (1R,2R,5R,8S,9S)-1,9,10,11,12,12-hexachloro-4,6-dioxa-5lambda4-thiatricyclo[7.2.1.0^{2,8}]dodec-10-en-5-one |
|---|
| Traditional Name | (1R,2R,5R,8S,9S)-1,9,10,11,12,12-hexachloro-4,6-dioxa-5lambda4-thiatricyclo[7.2.1.0^{2,8}]dodec-10-en-5-one |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@]12CO[S@](=O)OC[C@@]1([H])[C@]1(Cl)C(Cl)=C(Cl)[C@@]2(Cl)C1(Cl)Cl |
|---|
| InChI Identifier | InChI=1S/C9H6Cl6O3S/c10-5-6(11)8(13)4-2-18-19(16)17-1-3(4)7(5,12)9(8,14)15/h3-4H,1-2H2/t3-,4+,7+,8-,19- |
|---|
| InChI Key | RDYMFSUJUZBWLH-FYHLGZKHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sulfite esters. These are organic compounds containing an organic group attached to the sulfite oxoanion, with the formula R[SO3]2-. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organic oxoanionic compounds |
|---|
| Sub Class | Organic sulfites |
|---|
| Direct Parent | Sulfite esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sulfite ester
- Oxacycle
- Chloroalkene
- Haloalkene
- Organoheterocyclic compound
- Vinyl halide
- Vinyl chloride
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organochloride
- Organohalogen compound
- Alkyl halide
- Alkyl chloride
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0021900000-756f585a64cbe55e7daa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0079-0009000000-786e58cb3da4267cf13f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006t-1095000000-e45b8a4df54560630844 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0001900000-7f72e4f1c3b629bc3d8c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0095300000-5a2cb2c6d84ef88b7111 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uy0-2009000000-98c2ce5ecda62757ca07 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000900000-2eefcdeb338309d303ac | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0000900000-3af0d34b073e106e18b4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uk9-0009500000-ab8a8b8f97f0c283dcff | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000900000-e2d19f5f0adc47d59a36 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-4000900000-91cde5248808f8d1e494 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ue9-7000900000-5cdcfa6eb006761f93ee | View in MoNA |
|---|
|
|---|