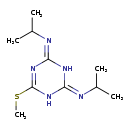| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-11-08 19:40:07 UTC |
|---|
| Update Date | 2020-06-04 23:01:12 UTC |
|---|
| BMDB ID | BMDB0063633 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Prometryn |
|---|
| Description | Prometryn is a diamino-1,3,5-triazine that is N,N'-di(propan-2-yl)-1,3,5-triazine-2,4-diamine substituted by a methylsulfanediyl group at position 6. It has a role as a herbicide, a xenobiotic and an environmental contaminant. It is a diamino-1,3,5-triazine and a methylthio-1,3,5-triazine. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(Methylthio)-4,6-bis(isopropylamino)-S-triazine | ChEBI | | N,N'-bis(1-methylethyl)-6-methylthio-1,3,5-triazine-2,4-diamine | ChEBI | | N,N'-diisopropyl-6-(methylthio)-1,3,5-triazine-2,4-diamine | ChEBI | | Prometryne | ChEBI | | Gesaguard 50 | MeSH | | 50, Gesaguard | MeSH |
|
|---|
| Chemical Formula | C10H19N5S |
|---|
| Average Molecular Weight | 241.356 |
|---|
| Monoisotopic Molecular Weight | 241.136116323 |
|---|
| IUPAC Name | N-[4-(methylsulfanyl)-6-[(propan-2-yl)imino]-1,2,5,6-tetrahydro-1,3,5-triazin-2-ylidene]propan-2-amine |
|---|
| Traditional Name | N-[4-(isopropylimino)-6-(methylsulfanyl)-3,5-dihydro-1,3,5-triazin-2-ylidene]propan-2-amine |
|---|
| CAS Registry Number | 7287-19-6 |
|---|
| SMILES | CSC1=NC(NC(N1)=NC(C)C)=NC(C)C |
|---|
| InChI Identifier | InChI=1S/C10H19N5S/c1-6(2)11-8-13-9(12-7(3)4)15-10(14-8)16-5/h6-7H,1-5H3,(H2,11,12,13,14,15) |
|---|
| InChI Key | AAEVYOVXGOFMJO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as methylthio-s-triazines. These are aromatic compounds containing a 1,3,5-triazine ring that is substituted at the 2-position with a methylthio group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Triazines |
|---|
| Sub Class | 1,3,5-triazines |
|---|
| Direct Parent | Methylthio-s-triazines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Methylthio-s-triazine
- 2,4-diamine-s-triazine
- Alkyl-2-thio-s-triazine
- Aryl thioether
- Amino-1,3,5-triazine
- Aminotriazine
- Secondary aliphatic/aromatic amine
- Alkylarylthioether
- N-aliphatic s-triazine
- Heteroaromatic compound
- Azacycle
- Sulfenyl compound
- Thioether
- Secondary amine
- Amine
- Hydrocarbon derivative
- Organosulfur compound
- Organonitrogen compound
- Organopnictogen compound
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000y-6950000000-96a3018d705b291169ac | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-0udi-0290000000-bf26c335678f721cba6c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 80V, Positive | splash10-0a4i-2900000000-b02036f70129cabcac55 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0006-0090000000-2d8117a2bc3a0e8508be | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-0k96-0290000000-70771d6bd91beb6c96ed | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-0a4i-1930000000-ac77e19da56eb40503ac | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 55V, Positive | splash10-0pb9-0960000000-83e13e34bcec40913933 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-0udi-0290000000-0aea2c6390c520cffab7 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0006-0090000000-f7f4e7ae1ec99d9833b1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-0udi-0290000000-2ccc2327871d90ee7c52 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-0006-0090000000-db644f7f7d9e9e260a16 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-0a4i-3900000000-d7fcf03d89127f4a4700 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-014i-9300000000-72558cc9c07c6d253fc9 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-066r-8900000000-cceba0065eade9968187 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-066r-8900000000-4f0db0e76a4f88e72d91 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-0a4i-1930000000-1879a0e9a96288696128 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-0k96-0390000000-2be8d9a95bb85fddeb30 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-0aor-3900000000-9d306dc26823070a6ae2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 55V, Positive | splash10-0udi-0290000000-706114a823dd644790bd | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-0a4i-2910000000-2c674b7fb5246f96c401 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-1090000000-6815701ba115a178a975 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-1790000000-f44f4d60f4e646d6c661 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9500000000-c0613ff8bd1190580cdc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-2790000000-99f33e82be7e02e08fda | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-9210000000-937bfccccafe6d55520d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-9800000000-700d34969d6d309c0153 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-0006-9650000000-142adcdeed30c354ebde | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100.40 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|