| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-12-04 20:29:17 UTC |
|---|
| Update Date | 2020-06-04 20:43:52 UTC |
|---|
| BMDB ID | BMDB0063638 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Lincomycin |
|---|
| Description | Lincomycin is an antibiotic produced by Streptomyces lincolnensis var. lincolnensis. It has been used in the treatment of staphylococcal, streptococcal, and Bacteroides fragilis infections (PubChem). Lincomycin inhibits protein synthesis in susceptible bacteria by binding to the 50S subunits of bacterial ribosomes and preventing peptide bond formation upon transcription. It is usually considered bacteriostatic, but may be bactericidal in high concentrations or when used against highly susceptible organisms. |
|---|
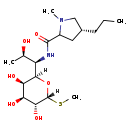
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| LCM | HMDB | | Lincomycine | HMDB | | Lincomyocin | HMDB | | Epilincomycin | HMDB | | Hemihydrate lincomycin monohydrochloride | HMDB | | Lincocin | HMDB | | Lincolnensin | HMDB | | Lincomycin a | HMDB | | Lincomycin hydrochloride | HMDB | | Lincomycin monohydrochloride | HMDB | | Lincomycin monohydrochloride, (2S-cis)-isomer | HMDB | | Lincomycin monohydrochloride, (L-threo)-isomer | HMDB | | Lincomycin monohydrochloride, hemihydrate | HMDB | | Lincomycin, (2S-cis)-isomer | HMDB | | Lincomycin, (L-threo)-isomer | HMDB |
|
|---|
| Chemical Formula | C18H34N2O6S |
|---|
| Average Molecular Weight | 406.537 |
|---|
| Monoisotopic Molecular Weight | 406.21375752 |
|---|
| IUPAC Name | (4R)-N-[(1R,2R)-2-hydroxy-1-[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(methylsulfanyl)oxan-2-yl]propyl]-1-methyl-4-propylpyrrolidine-2-carboxamide |
|---|
| Traditional Name | lincomycin |
|---|
| CAS Registry Number | 154-21-2 |
|---|
| SMILES | [H][C@@]1(O[C@]([H])([C@H](NC(=O)C2C[C@@H](CCC)CN2C)[C@@H](C)O)[C@H](O)[C@H](O)[C@H]1O)SC |
|---|
| InChI Identifier | InChI=1S/C18H34N2O6S/c1-5-6-10-7-11(20(3)8-10)17(25)19-12(9(2)21)16-14(23)13(22)15(24)18(26-16)27-4/h9-16,18,21-24H,5-8H2,1-4H3,(H,19,25)/t9-,10-,11?,12-,13+,14-,15-,16-,18-/m1/s1 |
|---|
| InChI Key | OJMMVQQUTAEWLP-ISVUEQNNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as proline and derivatives. Proline and derivatives are compounds containing proline or a derivative thereof resulting from reaction of proline at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Proline and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Proline or derivatives
- Alpha-amino acid amide
- Glycosyl compound
- S-glycosyl compound
- Pyrrolidine carboxylic acid or derivatives
- Pyrrolidine-2-carboxamide
- Monosaccharide
- Oxane
- N-alkylpyrrolidine
- Monothioacetal
- Pyrrolidine
- Tertiary aliphatic amine
- Tertiary amine
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxamide group
- Azacycle
- Organoheterocyclic compound
- Oxacycle
- Sulfenyl compound
- Polyol
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Organopnictogen compound
- Organic oxygen compound
- Organic oxide
- Carbonyl group
- Amine
- Organic nitrogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 2.93e+01 g/L | Not Available | | LogP | 0.56 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-2913000000-d125ca442f4c601e9a39 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-00c0-9602106000-31350d9eb8fe0368bfa6 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0319200000-139ddcba34e19650ea5f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-2931000000-bed26638dffce4e95302 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-7910000000-7d724ec0afa72d0b45a1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4s-6292200000-03967da3887e930924aa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ap1-9213000000-00ec697880a56054289f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kg-9531000000-92262505990e7631d58a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0900700000-cd9a0a26391bdfe078b3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0059-5911100000-5d8c7fc41772fd9bab9d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01pp-9100000000-217fb752e33a908a4028 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0014900000-d2789e972b45dee526dc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-9464100000-75f0ff15f86ac44459c1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9200000000-d2d2ba061cb2b04d9977 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| General References | - Cristina Juan, Juan Carlos Moltó, Jordi Mañes, Guillermina Font (2010). Cristina Juan, Juan Carlos Moltó, Jordi Mañes, Guillermina Font. Determination of macrolide and lincosamide antibiotics by pressurised liquid extraction and liquid chromatography-tandem mass spectrometry in meat and milk. Volume 21, Issue 12, Supplement, December 2010, Pages 1703-1709. Food Control.
|
|---|