| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-12-04 20:32:53 UTC |
|---|
| Update Date | 2020-06-04 20:46:20 UTC |
|---|
| BMDB ID | BMDB0063640 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Tetracycline |
|---|
| Description | Tetracycline is a broad spectrum polyketide antibiotic produced by the Streptomyces genus of Actinobacteria. It exerts a bacteriostatic effect on bacteria by binding reversible to the bacterial 30S ribosomal subunit and blocking incoming aminoacyl tRNA from binding to the ribosome acceptor site. It also binds to some extent to the bacterial 50S ribosomal subunit and may alter the cytoplasmic membrane causing intracellular components to leak from bacterial cells. |
|---|
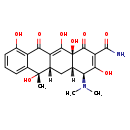
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (4S,4AS,5as,12as)-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide | ChEBI | | Abramycin | ChEBI | | Achromycin | ChEBI | | Anhydrotetracycline | ChEBI | | Deschlorobiomycin | ChEBI | | Liquamycin | ChEBI | | Tetracyclin | ChEBI | | Tetracyclinum | ChEBI | | Tetrazyklin | ChEBI | | Tsiklomitsin | ChEBI | | TC | Kegg | | Sumycin | Kegg | | Tetracycline HCL | HMDB |
|
|---|
| Chemical Formula | C22H24N2O8 |
|---|
| Average Molecular Weight | 444.4346 |
|---|
| Monoisotopic Molecular Weight | 444.153265754 |
|---|
| IUPAC Name | (4S,4aS,5aS,6S,12aS)-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide |
|---|
| Traditional Name | tetracycline |
|---|
| CAS Registry Number | 60-54-8 |
|---|
| SMILES | [H][C@@]12C[C@@]3([H])C(=C(O)[C@]1(O)C(=O)C(C(N)=O)=C(O)[C@H]2N(C)C)C(=O)C1=C(O)C=CC=C1[C@@]3(C)O |
|---|
| InChI Identifier | InChI=1S/C22H24N2O8/c1-21(31)8-5-4-6-11(25)12(8)16(26)13-9(21)7-10-15(24(2)3)17(27)14(20(23)30)19(29)22(10,32)18(13)28/h4-6,9-10,15,25,27-28,31-32H,7H2,1-3H3,(H2,23,30)/t9-,10-,15-,21+,22-/m0/s1 |
|---|
| InChI Key | OFVLGDICTFRJMM-WESIUVDSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetracyclines. These are polyketides having an octahydrotetracene-2-carboxamide skeleton, substituted with many hydroxy and other groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Tetracyclines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Tetracyclines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetracycline
- Naphthacene
- Tetracene
- Anthracene carboxylic acid or derivatives
- Tetralin
- Aryl ketone
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Cyclohexenone
- Aralkylamine
- Benzenoid
- Tertiary alcohol
- Vinylogous acid
- Tertiary aliphatic amine
- Amino acid or derivatives
- Tertiary amine
- Carboxamide group
- Primary carboxylic acid amide
- Ketone
- Polyol
- Carboxylic acid derivative
- Enol
- Amine
- Organic oxygen compound
- Organopnictogen compound
- Organonitrogen compound
- Organooxygen compound
- Alcohol
- Carbonyl group
- Organic nitrogen compound
- Organic oxide
- Hydrocarbon derivative
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 165 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 1.33e+00 g/L | Not Available | | LogP | -0.3 | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0bvi-5259500000-bd5eacef407c7cbcfc2a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0002-5023749000-5781179b3d4ec0f18e56 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_4_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_4_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_4_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_4_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_4_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_4_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_5_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_5_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_5_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_5_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Tetracycline,2TMS,#2" TMS) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-01sc-1900000000-7b792268aeb0a63c32c5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-01p9-1900000000-da0b69e206bb0369723c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-000i-3900000000-299e4496b1f8e2f213cc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-01ta-0000900000-e5ca6594fe72eb2bcd1f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0ik9-0402900000-9ef4a0d08180ce9b2a1c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0ik9-0402900000-a84ce5e2767545f4dd7e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-01sc-1900000000-7b792268aeb0a63c32c5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-01ta-0000900000-908fcaa876a610a2ecbe | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 45V, Negative | splash10-01p9-1900000000-da0b69e206bb0369723c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-0000900000-b0b8239482dc0ab660e1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01t9-0001900000-11d8380f89252d777068 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0btc-2268900000-9b20bc13399f86c0ff4a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0002900000-bbde63c16f1d6207b45a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fal-0118900000-1d65f8ee8fa0af312c20 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00r6-9166000000-84828062c581777db668 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0000900000-3355d21a81297e70a696 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0000900000-aec34c9f29d5643c9dd7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-015i-9333100000-24fa0a1d06d44e57511d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002f-0004900000-ce47e08c76771852979b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05ec-0049700000-8b1cbde084e892a26b15 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001u-1069300000-553a5cff4aef98461f76 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| General References | - Nina Bilandžić, Božica Solomun Kolanović, Ivana Varenina, Giampiero Scortichini, Loredana Annunziata, Matko Brstilo, Nevenka Rudan (2011). Nina Bilandžić, Božica Solomun Kolanović, Ivana Varenina, Giampiero Scortichini, Loredana Annunziata, Matko Brstilo, Nevenka Rudan. Veterinary drug residues determination in raw milk in Croatia. Food Control. Volume 22, Issue 12, December 2011, Pages 1941-1948. Food Control.
|
|---|