| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-02-10 19:47:47 UTC |
|---|
| Update Date | 2020-06-04 23:02:10 UTC |
|---|
| BMDB ID | BMDB0063723 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
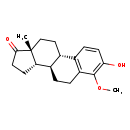
| Common Name | 4-methoxyestrone |
|---|
| Description | Thyrotropin releasing hormone, also known as TRH or protirelin, belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. Thyrotropin releasing hormone is a very strong basic compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| L-Pyroglutamyl-L-histidyl-L-prolineamide | ChEBI | | Thyroliberin | ChEBI | | Thyrotropic releasing hormone | ChEBI | | Thyrotropic-releasing factor | ChEBI | | Thyrotropin-releasing factor | ChEBI | | TRH | ChEBI | | TSH-Releasing factor | ChEBI | | TSH-Releasing hormone | ChEBI | | Protirelin | Kegg | | Relefact TRH | Kegg | | Abbott brand OF protirelin | HMDB | | Abbott-38579 | HMDB | | Antepan | HMDB | | Aventis brand OF protirelin | HMDB | | Novartis brand OF protirelin | HMDB | | Proterelin tartrate | HMDB | | Proterelin tartrate hydrate | HMDB | | Protirelin abbott brand | HMDB | | Protirelin aventis brand | HMDB | | Stimu TSH | HMDB | | Tartrate hydrate, proterelin | HMDB | | Thypinone | HMDB | | Abbott 38579 | HMDB | | Protirelin tartrate (1:1) | HMDB | | TRH Ferring | HMDB | | TRH Prem | HMDB | | Thyrotropin-releasing hormone | HMDB | | Thyrotropin-releasing hormone tartrate | HMDB | | Abbott38579 | HMDB | | Ferring brand OF protirelin | HMDB | | Henning berlin brand OF protirelin | HMDB | | Hydrate, proterelin tartrate | HMDB | | Merck brand OF protirelin | HMDB | | Prem, TRH | HMDB | | Protirelin ferring brand | HMDB | | Protirelin merck brand | HMDB | | Stimu-TSH | HMDB | | Thyroliberin TRH merck | HMDB | | Thyrotropin releasing factor | HMDB | | Protirelin novartis brand | HMDB | | StimuTSH | HMDB | | TRH, Relefact | HMDB | | Thyrotropin releasing hormone tartrate | HMDB |
|
|---|
| Chemical Formula | C19H24O3 |
|---|
| Average Molecular Weight | 300.3921 |
|---|
| Monoisotopic Molecular Weight | 300.172544634 |
|---|
| IUPAC Name | (1S,10R,11S,15R)-5-hydroxy-6-methoxy-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-trien-14-one |
|---|
| Traditional Name | (1S,10R,11S,15R)-5-hydroxy-6-methoxy-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-trien-14-one |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | COC1=C(O)C=CC2=C1CC[C@H]1[C@@H]3CCC(=O)[C@]3(C)CC[C@H]21 |
|---|
| InChI Identifier | InChI=1S/C19H24O3/c1-19-10-9-12-11-5-7-16(20)18(22-2)14(11)4-3-13(12)15(19)6-8-17(19)21/h5,7,12-13,15,20H,3-4,6,8-10H2,1-2H3/t12-,13-,15+,19-/m1/s1 |
|---|
| InChI Key | PUEXVLNGOBYUEW-PITQQHRWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- Histidine or derivatives
- N-acyl-alpha amino acid or derivatives
- Proline or derivatives
- Alpha-amino acid amide
- Alpha-amino acid or derivatives
- N-acylpyrrolidine
- Pyrrolidine carboxylic acid or derivatives
- Pyrrolidine-2-carboxamide
- Pyrrolidone
- 2-pyrrolidone
- Azole
- Imidazole
- Heteroaromatic compound
- Pyrrolidine
- Tertiary carboxylic acid amide
- Primary carboxylic acid amide
- Lactam
- Carboxamide group
- Secondary carboxylic acid amide
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fe0-0490000000-e0b809016b8c8bad412a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-08ou-1059000000-fc0f62cbcdf1d5df5108 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0029000000-d7fd1e3ab4e4be728914 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0v4i-0792000000-557e318f749c8060ba9b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pi9-5490000000-42c39c3194e527bdc751 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-0655540f59ad5c8c43a0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-5f7c58555ff69013e200 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0kec-1090000000-461b8b04617809255fd7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-dd82c6133109d0e6e28e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00l2-0090000000-66e609af1e31bb237106 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kb-0090000000-fc050b7ed710552a3f97 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ue9-0049000000-98a596f43e468044b417 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f89-5794000000-a3bbd4498b460f1ededa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f6t-0960000000-f8b5386627dfc6028664 | View in MoNA |
|---|
|
|---|