| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-03 19:35:36 UTC |
|---|
| Update Date | 2020-04-22 15:56:32 UTC |
|---|
| BMDB ID | BMDB0064011 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Methionyl-Threonine |
|---|
| Description | Methionyl-Threonine, also known as m-T dipeptide or met-THR, belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. Based on a literature review very few articles have been published on Methionyl-Threonine. |
|---|
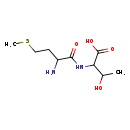
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| L-Methionyl-L-threonine | HMDB | | m-T Dipeptide | HMDB | | Met-THR | HMDB | | Methionine threonine dipeptide | HMDB | | Methionine-threonine dipeptide | HMDB | | Methionylthreonine | HMDB | | MT Dipeptide | HMDB | | 2-{[2-amino-1-hydroxy-4-(methylsulfanyl)butylidene]amino}-3-hydroxybutanoate | HMDB | | 2-{[2-amino-1-hydroxy-4-(methylsulphanyl)butylidene]amino}-3-hydroxybutanoate | HMDB | | 2-{[2-amino-1-hydroxy-4-(methylsulphanyl)butylidene]amino}-3-hydroxybutanoic acid | HMDB |
|
|---|
| Chemical Formula | C9H18N2O4S |
|---|
| Average Molecular Weight | 250.315 |
|---|
| Monoisotopic Molecular Weight | 250.098727764 |
|---|
| IUPAC Name | 2-[2-amino-4-(methylsulfanyl)butanamido]-3-hydroxybutanoic acid |
|---|
| Traditional Name | 2-[2-amino-4-(methylsulfanyl)butanamido]-3-hydroxybutanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CSCCC(N)C(=O)NC(C(C)O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C9H18N2O4S/c1-5(12)7(9(14)15)11-8(13)6(10)3-4-16-2/h5-7,12H,3-4,10H2,1-2H3,(H,11,13)(H,14,15) |
|---|
| InChI Key | KAKJTZWHIUWTTD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Dipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-dipeptide
- Methionine or derivatives
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- Alpha-amino acid amide
- Alpha-amino acid or derivatives
- Beta-hydroxy acid
- Short-chain hydroxy acid
- Fatty acyl
- Fatty acid
- Fatty amide
- Hydroxy acid
- N-acyl-amine
- Amino acid or derivatives
- Secondary carboxylic acid amide
- Secondary alcohol
- Amino acid
- Carboxamide group
- Carboxylic acid
- Dialkylthioether
- Sulfenyl compound
- Monocarboxylic acid or derivatives
- Thioether
- Organic oxygen compound
- Primary aliphatic amine
- Organic nitrogen compound
- Alcohol
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organopnictogen compound
- Amine
- Primary amine
- Organosulfur compound
- Organonitrogen compound
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f7p-9320000000-d1d28a932bc175d2d3c8 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0097-9302000000-f82e3319135bd13a4a5e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f89-0490000000-74bff564597f6fec04be | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udr-5930000000-74ab442a3e375c0212e8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zfu-9300000000-d76edcac6d59971e4817 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-4290000000-feaafc8f39aae81dd72e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-9430000000-2f20fc00505f05a60379 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9100000000-42d18235d24cba46d727 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0390000000-1957ae25c8ca33ae72ec | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0zfr-5920000000-486b23fd951fcf3b05f7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0r09-9200000000-b5b1169d36e401d03963 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000t-0190000000-7674abb2a3af9069d72c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-9670000000-c7bb74b492f15b93b3ff | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9000000000-ae4cdd0277ba04e9811d | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|