| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-03 19:40:36 UTC |
|---|
| Update Date | 2020-03-13 17:50:40 UTC |
|---|
| BMDB ID | BMDB0064097 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Tryptophylhydroxyproline |
|---|
| Description | Tryptophylhydroxyproline, also known as W-HP dipeptide or TRP-hpro, belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. Based on a literature review very few articles have been published on Tryptophylhydroxyproline. |
|---|
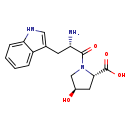
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Tryptophan hydroxyproline dipeptide | HMDB | | L-Tryptophyl-L-hydroxyproline | HMDB | | TRP-HPro | HMDB | | Tryptophan-hydroxyproline dipeptide | HMDB | | W-HP Dipeptide | HMDB | | WHP Dipeptide | HMDB | | TRP-Hyp | HMDB | | L-TRP-L-Hyp | HMDB | | Tryptophyl-hydroxyproline | HMDB | | (2S,4R)-1-[(2S)-2-Amino-3-(1H-indol-3-yl)propanoyl]-4-hydroxypyrrolidine-2-carboxylate | HMDB | | Tryptophylhydroxyproline | HMDB |
|
|---|
| Chemical Formula | C16H19N3O4 |
|---|
| Average Molecular Weight | 317.345 |
|---|
| Monoisotopic Molecular Weight | 317.137556104 |
|---|
| IUPAC Name | (2S,4R)-1-[(2S)-2-amino-3-(1H-indol-3-yl)propanoyl]-4-hydroxypyrrolidine-2-carboxylic acid |
|---|
| Traditional Name | (2S,4R)-1-[(2S)-2-amino-3-(1H-indol-3-yl)propanoyl]-4-hydroxypyrrolidine-2-carboxylic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | N[C@@H](CC1=CNC2=CC=CC=C12)C(=O)N1C[C@H](O)C[C@H]1C(O)=O |
|---|
| InChI Identifier | InChI=1S/C16H19N3O4/c17-12(5-9-7-18-13-4-2-1-3-11(9)13)15(21)19-8-10(20)6-14(19)16(22)23/h1-4,7,10,12,14,18,20H,5-6,8,17H2,(H,22,23)/t10-,12+,14+/m1/s1 |
|---|
| InChI Key | XMEUQXQNFRSXLF-OSMZGAPFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Dipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-dipeptide
- Proline or derivatives
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- N-acyl-l-alpha-amino acid
- Alpha-amino acid amide
- Triptan
- Alpha-amino acid or derivatives
- 3-alkylindole
- Indole
- Indole or derivatives
- N-acylpyrrolidine
- Pyrrolidine carboxylic acid
- Pyrrolidine carboxylic acid or derivatives
- Aralkylamine
- Substituted pyrrole
- Benzenoid
- Heteroaromatic compound
- Tertiary carboxylic acid amide
- Pyrrolidine
- Pyrrole
- Secondary alcohol
- Amino acid or derivatives
- Carboxamide group
- Amino acid
- Azacycle
- Organoheterocyclic compound
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic oxygen compound
- Hydrocarbon derivative
- Primary aliphatic amine
- Organic oxide
- Organopnictogen compound
- Alcohol
- Organonitrogen compound
- Carbonyl group
- Organooxygen compound
- Amine
- Primary amine
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0209000000-3d4ae2c12dea4fdab8f1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05mo-0902000000-4987df87b9ff1f6ea4ee | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-0900000000-7b9f6b4edc5922dd7854 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00kb-0296000000-b67a27ee6aaf400269d8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03xr-1922000000-db28d97fd64c94fbff40 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001l-4900000000-57794d687bce85a3e438 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|