| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-03 19:41:54 UTC |
|---|
| Update Date | 2020-04-22 15:57:12 UTC |
|---|
| BMDB ID | BMDB0064119 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
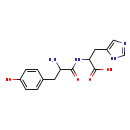
| Common Name | Tyrosyl-Histidine |
|---|
| Description | Tyrosyl-Histidine, also known as y-H dipeptide or tyr-his, belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. Based on a literature review very few articles have been published on Tyrosyl-Histidine. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| L-Tyrosyl-L-histidine | HMDB | | Tyr-his | HMDB | | Tyrosine histidine dipeptide | HMDB | | Tyrosine-histidine dipeptide | HMDB | | Tyrosylhistidine | HMDB | | Y-H dipeptide | HMDB | | YH dipeptide | HMDB | | 2-{[2-amino-1-hydroxy-3-(4-hydroxyphenyl)propylidene]amino}-3-(1H-imidazol-5-yl)propanoate | HMDB |
|
|---|
| Chemical Formula | C15H18N4O4 |
|---|
| Average Molecular Weight | 318.3278 |
|---|
| Monoisotopic Molecular Weight | 318.132805084 |
|---|
| IUPAC Name | 2-[2-amino-3-(4-hydroxyphenyl)propanamido]-3-(1H-imidazol-5-yl)propanoic acid |
|---|
| Traditional Name | 2-[2-amino-3-(4-hydroxyphenyl)propanamido]-3-(3H-imidazol-4-yl)propanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | NC(CC1=CC=C(O)C=C1)C(=O)NC(CC1=CN=CN1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C15H18N4O4/c16-12(5-9-1-3-11(20)4-2-9)14(21)19-13(15(22)23)6-10-7-17-8-18-10/h1-4,7-8,12-13,20H,5-6,16H2,(H,17,18)(H,19,21)(H,22,23) |
|---|
| InChI Key | ZQOOYCZQENFIMC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Dipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-dipeptide
- Tyrosine or derivatives
- Phenylalanine or derivatives
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid amide
- Alpha-amino acid or derivatives
- Amphetamine or derivatives
- Medium-chain fatty acid
- Hydroxy fatty acid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Amino fatty acid
- Aralkylamine
- Fatty amide
- Monocyclic benzene moiety
- Benzenoid
- Fatty acid
- Fatty acyl
- Secondary carboxylic acid amide
- Carboxamide group
- Amino acid or derivatives
- Amino acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Organic nitrogen compound
- Primary aliphatic amine
- Organonitrogen compound
- Organooxygen compound
- Carbonyl group
- Primary amine
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Amine
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01q0-5910000000-b254c42bf24e16941f26 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-000j-2905000000-7aac9d9de99ae7f3d89e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0gbi-0729000000-ee68a16346a8f4766c6e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-029i-0910000000-a1f5d97679b9d1a9a05c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-3900000000-e66920aa63eb7b8eb505 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0149000000-520f86eee5095340a448 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0wt9-2962000000-dfdbe7176a62063277f5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-11c9-6900000000-5f93a5e4722fe180f090 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0709000000-6ceb90ac267e1c978d1e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0rl0-3911000000-7f843e005f61e25b838c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-9820000000-8d7ae73874a9e86fe8af | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014r-0409000000-aa0de16108e647b0ff3a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0900000000-1eb94dc0f76cf4699a23 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-060d-5900000000-0cc1729748d365b45ddc | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|