| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-04 17:42:24 UTC |
|---|
| Update Date | 2020-04-22 16:09:23 UTC |
|---|
| BMDB ID | BMDB0066047 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | PE(DiMe(11,5)/DiMe(13,5)) |
|---|
| Description | Not Available |
|---|
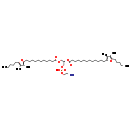
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| PE(DiMe(11,5)/DiMe(13,5)) | SMPDB | | Phosphatidylethanolamine(11D5/13D5) | SMPDB | | Phosphatidylethanolamine(DiMe(11,5)/DiMe(13,5)) | SMPDB | | PE(11D5/13D5) | SMPDB |
|
|---|
| Chemical Formula | C51H90NO10P |
|---|
| Average Molecular Weight | 908.2348 |
|---|
| Monoisotopic Molecular Weight | 907.630234617 |
|---|
| IUPAC Name | (2-aminoethoxy)[(2R)-2-{[13-(3,4-dimethyl-5-pentylfuran-2-yl)tridecanoyl]oxy}-3-{[11-(3,4-dimethyl-5-pentylfuran-2-yl)undecanoyl]oxy}propoxy]phosphinic acid |
|---|
| Traditional Name | 2-aminoethoxy((2R)-2-{[13-(3,4-dimethyl-5-pentylfuran-2-yl)tridecanoyl]oxy}-3-{[11-(3,4-dimethyl-5-pentylfuran-2-yl)undecanoyl]oxy}propoxy)phosphinic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCCCCC1=C(C)C(C)=C(CCCCCCCCCCCCC(=O)O[C@H](COC(=O)CCCCCCCCCCC2=C(C)C(C)=C(CCCCC)O2)COP(O)(=O)OCCN)O1 |
|---|
| InChI Identifier | InChI=1S/C51H90NO10P/c1-7-9-25-31-46-41(3)43(5)48(61-46)33-27-21-17-13-11-12-14-20-24-30-36-51(54)60-45(40-59-63(55,56)58-38-37-52)39-57-50(53)35-29-23-19-16-15-18-22-28-34-49-44(6)42(4)47(62-49)32-26-10-8-2/h45H,7-40,52H2,1-6H3,(H,55,56)/t45-/m1/s1 |
|---|
| InChI Key | ITYKVNCCHSCSRQ-WBVITSLISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | This compound belongs to the class of organic compounds known as phosphatidylethanolamines. These are glycerophosphoetahnolamines in which two fatty acids are bonded to the glycerol moiety through ester linkages. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphoethanolamines |
|---|
| Direct Parent | Phosphatidylethanolamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diacylglycero-3-phosphoethanolamine
- Furanoid fatty acid
- Phosphoethanolamine
- Fatty acid ester
- Dialkyl phosphate
- Dicarboxylic acid or derivatives
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Fatty acyl
- Heteroaromatic compound
- Furan
- Carboxylic acid ester
- Amino acid or derivatives
- Oxacycle
- Carboxylic acid derivative
- Organoheterocyclic compound
- Carbonyl group
- Organonitrogen compound
- Primary aliphatic amine
- Organooxygen compound
- Organic oxygen compound
- Organic nitrogen compound
- Amine
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxide
- Primary amine
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Intracellular membrane
|
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-9004020011-272f891edf4b2df2261a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9102000010-690831f414993030d2e2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9003101200-a92a7d6f1a1ac32bf3d0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-055b-1109020011-93a7485e8d7d0d9875cc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004m-5509010010-f05eb0bf554414e7af14 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01t9-9202000000-4f89dda644a289b99aca | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0003000009-2bb2f3607f0cf7b52a69 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0003000009-2bb2f3607f0cf7b52a69 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-054k-0109030003-f2495de9751382fc56ca | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000000119-404a8294355aaea2b726 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0aor-0000360916-132aa0dc464ed21dd048 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066r-0000360911-4045ce1da8342d5d7c5e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0000000109-96242732c32fd5639f51 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001r-0000000229-d2fa6b6c809a4c1e3c58 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0100030191-3dc16440658cd69c033a | View in MoNA |
|---|
|
|---|