| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-04 19:02:23 UTC |
|---|
| Update Date | 2020-05-21 16:29:09 UTC |
|---|
| BMDB ID | BMDB0066663 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
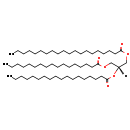
| Common Name | TG(20:0/18:0/17:0) |
|---|
| Description | TG(20:0/18:0/17:0) belongs to the family of triradyglycerols, which are glycerolipids lipids containing a common glycerol backbone to which at least one fatty acyl group is esterified. Their general formula is [R1]OCC(CO[R2])O[R3]. TG(20:0/18:0/17:0) is made up of one eicosanoyl(R1), one octadecanoyl(R2), and one heptadecanoyl(R3). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-arachidoyl-2-stearoyl-3-margaroyl-glycerol | SMPDB, HMDB | | TG(55:0) | SMPDB, HMDB | | Tag(20:0/18:0/17:0) | SMPDB, HMDB | | Tag(55:0) | SMPDB, HMDB | | Triacylglycerol(20:0/18:0/17:0) | SMPDB, HMDB | | Triacylglycerol(55:0) | SMPDB, HMDB | | Triacylglycerol | SMPDB, HMDB | | Triglyceride | SMPDB, HMDB | | TG(20:0/18:0/17:0) | SMPDB | | Triacylglycerol | Lipid Annotator | | 1-arachidonyl-2-stearoyl-3-heptadecanoyl-glycerol | Lipid Annotator, HMDB | | TAG(20:0/18:0/17:0) | Lipid Annotator | | TAG(55:0) | Lipid Annotator | | Tracylglycerol(55:0) | Lipid Annotator, HMDB | | TG(55:0) | Lipid Annotator | | 1-eicosanoyl-2-octadecanoyl-3-margaroyl-glycerol | Lipid Annotator, HMDB | | Tracylglycerol(20:0/18:0/17:0) | Lipid Annotator, HMDB |
|
|---|
| Chemical Formula | C58H112O6 |
|---|
| Average Molecular Weight | 905.528 |
|---|
| Monoisotopic Molecular Weight | 904.845891326 |
|---|
| IUPAC Name | (2R)-3-(heptadecanoyloxy)-2-(octadecanoyloxy)propyl icosanoate |
|---|
| Traditional Name | (2R)-3-(heptadecanoyloxy)-2-(octadecanoyloxy)propyl icosanoate |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@](COC(=O)CCCCCCCCCCCCCCCC)(COC(=O)CCCCCCCCCCCCCCCCCCC)OC(=O)CCCCCCCCCCCCCCCCC |
|---|
| InChI Identifier | InChI=1S/C58H112O6/c1-4-7-10-13-16-19-22-25-28-29-31-33-36-39-42-45-48-51-57(60)63-54-55(53-62-56(59)50-47-44-41-38-35-32-27-24-21-18-15-12-9-6-3)64-58(61)52-49-46-43-40-37-34-30-26-23-20-17-14-11-8-5-2/h55H,4-54H2,1-3H3/t55-/m1/s1 |
|---|
| InChI Key | XQLZFNFYDZDZDH-KZRJWCEASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as triacylglycerols. These are glycerides consisting of three fatty acid chains covalently bonded to a glycerol molecule through ester linkages. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerolipids |
|---|
| Sub Class | Triradylcglycerols |
|---|
| Direct Parent | Triacylglycerols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triacyl-sn-glycerol
- Tricarboxylic acid or derivatives
- Fatty acid ester
- Fatty acyl
- Carboxylic acid ester
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0045015009-bb7c0ab2ca0c86c9d15f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0900-0069001000-4cce02ce00c1c61e684f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-07vi-2096001000-555d81ceb029722ba348 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000000009-e70e5c7e6252f8ee8c86 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0000000009-e70e5c7e6252f8ee8c86 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05g3-0000049003-ac97a3cbe46482a14b5e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0000000009-00c6dd835d7efaa7b0b6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0000000009-00c6dd835d7efaa7b0b6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-0000000009-00c6dd835d7efaa7b0b6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000000009-c705e8a6fbb38d1be275 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0000000009-c705e8a6fbb38d1be275 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05g3-0020049003-73546e3b44e1ffc1baee | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-5370013129-6c7fc19f75c6b00b6446 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0609-9350001110-d6feae18f35b1fa460c1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06dl-9588201000-501625358f2975573ac2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000000009-03016e1fa888e42abf74 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0000000009-03016e1fa888e42abf74 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-04mo-0004009004-b8eeb59d246676504998 | View in MoNA |
|---|
|
|---|