| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-04 19:27:19 UTC |
|---|
| Update Date | 2020-05-21 16:29:21 UTC |
|---|
| BMDB ID | BMDB0067827 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | TG(i-20:0/14:0/17:0) |
|---|
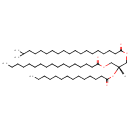
| Description | TG(i-20:0/14:0/17:0) belongs to the family of triradyglycerols, which are glycerolipids lipids containing a common glycerol backbone to which at least one fatty acyl group is esterified. Their general formula is [R1]OCC(CO[R2])O[R3]. TG(i-20:0/14:0/17:0) is made up of one 18-methylnonadecanoyl(R1), one tetradecanoyl(R2), and one heptadecanoyl(R3). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-isoeicosanoyl-2-myristoyl-3-margaroyl-glycerol | SMPDB, HMDB | | TG(i-20:0/14:0/17:0) | SMPDB | | TG(51:0) | SMPDB, HMDB | | Tag(i-20:0/14:0/17:0) | SMPDB, HMDB | | Tag(51:0) | SMPDB, HMDB | | Triacylglycerol(i-20:0/14:0/17:0) | SMPDB, HMDB | | Triacylglycerol(51:0) | SMPDB, HMDB | | Triacylglycerol | SMPDB, HMDB | | Triglyceride | SMPDB, HMDB | | 1-isoeicosanoyl-2-tetradecanoyl-3-margaroyl-glycerol | Lipid Annotator, HMDB | | Tracylglycerol(51:0) | Lipid Annotator, HMDB | | 1-isoeicosanoyl-2-myristoyl-3-heptadecanoyl-glycerol | Lipid Annotator, HMDB | | Tracylglycerol(i-20:0/14:0/17:0) | Lipid Annotator, HMDB |
|
|---|
| Chemical Formula | C54H104O6 |
|---|
| Average Molecular Weight | 849.42 |
|---|
| Monoisotopic Molecular Weight | 848.783291069 |
|---|
| IUPAC Name | (2R)-3-(heptadecanoyloxy)-2-(tetradecanoyloxy)propyl 18-methylnonadecanoate |
|---|
| Traditional Name | (2R)-3-(heptadecanoyloxy)-2-(tetradecanoyloxy)propyl 18-methylnonadecanoate |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@](COC(=O)CCCCCCCCCCCCCCCC)(COC(=O)CCCCCCCCCCCCCCCCC(C)C)OC(=O)CCCCCCCCCCCCC |
|---|
| InChI Identifier | InChI=1S/C54H104O6/c1-5-7-9-11-13-15-17-18-22-26-29-33-37-41-45-52(55)58-48-51(60-54(57)47-43-39-35-31-24-16-14-12-10-8-6-2)49-59-53(56)46-42-38-34-30-27-23-20-19-21-25-28-32-36-40-44-50(3)4/h50-51H,5-49H2,1-4H3/t51-/m1/s1 |
|---|
| InChI Key | QRTVGXOFSKZYSF-NLXJDERGSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as triacylglycerols. These are glycerides consisting of three fatty acid chains covalently bonded to a glycerol molecule through ester linkages. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerolipids |
|---|
| Sub Class | Triradylcglycerols |
|---|
| Direct Parent | Triacylglycerols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triacyl-sn-glycerol
- Tricarboxylic acid or derivatives
- Fatty acid ester
- Fatty acyl
- Carboxylic acid ester
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000000090-1106c63e15e17379080a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0000000090-1106c63e15e17379080a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ij-0000094030-096a2088737959a3685c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0i03-0092001010-bb79c1dfe558a8199ae3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-02t9-0093000000-53ad74e23c87e0702228 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-07vl-2092000000-10128257433780b03947 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-5340021290-eb09d5bae5ae3ace96a3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-08mi-9230010310-405b14ad6cc83e78707b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08fu-7493101000-012ed06ca68e3fa30766 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000000090-5410c14454c61279c57a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0000000090-5410c14454c61279c57a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0bf0-0009099090-993e6e3817068478bed8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000000090-f995cfd7a13a01e49dc2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0000000090-f995cfd7a13a01e49dc2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ij-0020094030-4cf526fa45f5d949c309 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000000090-365a203151f322c3d027 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0000000090-365a203151f322c3d027 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-0000000090-365a203151f322c3d027 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0063032090-37e2cb33be803f0308a7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0094010000-abc82ca94003c3a1774b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0cdi-1093000000-2e9f5409dab6bf16eb2e | View in MoNA |
|---|
|
|---|