| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-04 19:34:48 UTC |
|---|
| Update Date | 2020-05-21 16:29:11 UTC |
|---|
| BMDB ID | BMDB0068243 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | TG(21:0/14:0/17:0) |
|---|
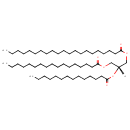
| Description | TG(21:0/14:0/17:0) belongs to the family of triradyglycerols, which are glycerolipids lipids containing a common glycerol backbone to which at least one fatty acyl group is esterified. Their general formula is [R1]OCC(CO[R2])O[R3]. TG(21:0/14:0/17:0) is made up of one heneicosanoyl(R1), one tetradecanoyl(R2), and one heptadecanoyl(R3). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-heneicosyloyl-2-myristoyl-3-margaroyl-glycerol | SMPDB, HMDB | | TG(52:0) | SMPDB, HMDB | | Tag(21:0/14:0/17:0) | SMPDB, HMDB | | Tag(52:0) | SMPDB, HMDB | | Triacylglycerol(21:0/14:0/17:0) | SMPDB, HMDB | | Triacylglycerol(52:0) | SMPDB, HMDB | | Triacylglycerol | SMPDB, HMDB | | Triglyceride | SMPDB, HMDB | | TG(21:0/14:0/17:0) | SMPDB | | 1-heneicosyloyl-2-tetradecanoyl-3-margaroyl-glycerol | Lipid Annotator, HMDB | | TG(52:0) | Lipid Annotator | | 1-heneicosyloyl-2-myristoyl-3-heptadecanoyl-glycerol | Lipid Annotator, HMDB | | Triacylglycerol | Lipid Annotator | | Tracylglycerol(21:0/14:0/17:0) | Lipid Annotator, HMDB | | TAG(52:0) | Lipid Annotator | | TAG(21:0/14:0/17:0) | Lipid Annotator | | Tracylglycerol(52:0) | Lipid Annotator, HMDB |
|
|---|
| Chemical Formula | C55H106O6 |
|---|
| Average Molecular Weight | 863.447 |
|---|
| Monoisotopic Molecular Weight | 862.798941133 |
|---|
| IUPAC Name | (2R)-3-(heptadecanoyloxy)-2-(tetradecanoyloxy)propyl henicosanoate |
|---|
| Traditional Name | (2R)-3-(heptadecanoyloxy)-2-(tetradecanoyloxy)propyl henicosanoate |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@](COC(=O)CCCCCCCCCCCCCCCC)(COC(=O)CCCCCCCCCCCCCCCCCCCC)OC(=O)CCCCCCCCCCCCC |
|---|
| InChI Identifier | InChI=1S/C55H106O6/c1-4-7-10-13-16-19-22-24-26-27-28-29-31-34-36-39-42-45-48-54(57)60-51-52(61-55(58)49-46-43-40-37-32-21-18-15-12-9-6-3)50-59-53(56)47-44-41-38-35-33-30-25-23-20-17-14-11-8-5-2/h52H,4-51H2,1-3H3/t52-/m1/s1 |
|---|
| InChI Key | ZQYBQGXZELRABE-OIVUAWODSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as triacylglycerols. These are glycerides consisting of three fatty acid chains covalently bonded to a glycerol molecule through ester linkages. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerolipids |
|---|
| Sub Class | Triradylcglycerols |
|---|
| Direct Parent | Triacylglycerols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triacyl-sn-glycerol
- Tricarboxylic acid or derivatives
- Fatty acid ester
- Fatty acyl
- Carboxylic acid ester
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0000000090-0b297ecc356d1befc4b1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0000000090-0b297ecc356d1befc4b1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000l-0000094030-9db5d2cd233c9033246a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0kxr-0095002010-af8863bbf68940f13b3e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05r0-0096000000-be92e4858f8e358ca148 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05r0-3094000000-3effb009a24d51046f32 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000000090-1faf87895f2770b7ee12 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0000000090-1faf87895f2770b7ee12 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0168-0009099090-2303561ec07d8113aabe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-4340021290-1e65ef8417a745ea6d24 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-08mi-9220000210-1c663a182831227c253c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08fu-7494101000-66ec4901044e39916820 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0000000090-93d8699dad5e4e368621 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0000000090-93d8699dad5e4e368621 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000l-0010094030-addb872b897743c30d5a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0000000090-07226c06cd19fb8c921b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0000000090-07226c06cd19fb8c921b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0000000090-07226c06cd19fb8c921b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0053033090-c5c0ba6460fc79bd99dc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a6r-0094010000-8c1bbc1ec30fe4f5ef4e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ar0-1094000000-d156517adb132a3099c1 | View in MoNA |
|---|
|
|---|