| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 16:59:34 UTC |
|---|
| Update Date | 2020-04-22 18:55:26 UTC |
|---|
| BMDB ID | BMDB0096000 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Neurotensin 11-13 |
|---|
| Description | Neurotensin 11-13 belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. Based on a literature review very few articles have been published on Neurotensin 11-13. |
|---|
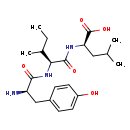
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Neurotensin (11-13) | HMDB | | Neurotensin fragment 11-13 | HMDB | | Tyr-ile-leu | HMDB | | (2R)-2-{[(2S)-2-{[(2R)-2-amino-1-hydroxy-3-(4-hydroxyphenyl)propylidene]amino}-1-hydroxy-3-methylpentylidene]amino}-4-methylpentanoate | Generator |
|
|---|
| Chemical Formula | C21H33N3O5 |
|---|
| Average Molecular Weight | 407.5038 |
|---|
| Monoisotopic Molecular Weight | 407.242021181 |
|---|
| IUPAC Name | (2R)-2-[(2S)-2-[(2R)-2-amino-3-(4-hydroxyphenyl)propanamido]-3-methylpentanamido]-4-methylpentanoic acid |
|---|
| Traditional Name | (2R)-2-[(2S)-2-[(2R)-2-amino-3-(4-hydroxyphenyl)propanamido]-3-methylpentanamido]-4-methylpentanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCC(C)[C@H](NC(=O)[C@H](N)CC1=CC=C(O)C=C1)C(=O)N[C@H](CC(C)C)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C21H33N3O5/c1-5-13(4)18(20(27)23-17(21(28)29)10-12(2)3)24-19(26)16(22)11-14-6-8-15(25)9-7-14/h6-9,12-13,16-18,25H,5,10-11,22H2,1-4H3,(H,23,27)(H,24,26)(H,28,29)/t13?,16-,17-,18+/m1/s1 |
|---|
| InChI Key | HVPPEXXUDXAPOM-WOFBESPQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- Tyrosine or derivatives
- Phenylalanine or derivatives
- Isoleucine or derivatives
- Proline or derivatives
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid amide
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- Amphetamine or derivatives
- Pyrrolidine carboxylic acid or derivatives
- Pyrrolidine-2-carboxamide
- N-acylpyrrolidine
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Fatty amide
- Benzenoid
- Fatty acyl
- Pyrrolidine
- Tertiary carboxylic acid amide
- Amino acid or derivatives
- Amino acid
- Carboxamide group
- Secondary carboxylic acid amide
- Organoheterocyclic compound
- Azacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Primary aliphatic amine
- Hydrocarbon derivative
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Organic oxide
- Organic nitrogen compound
- Amine
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-3912000000-dc75daa7f5f0787c6983 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-000i-4900100000-7509d817b86522047014 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-06rm-1955400000-f3d52ee6ef274b948570 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-4920000000-03f1247155187e465e4a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-5900000000-3346a2f263ca121b4b81 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0125900000-b6e3c35da186cfa880cc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08gr-3956100000-a486cf0bcb6fa8e5c192 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-4920000000-16dd637660d7ff064709 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a59-0940700000-b50b7e35abae7c4b1599 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01sl-3920000000-0a5a71a4303b0b31e6e7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0296-5900000000-353611c61a934bc135d1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-06rb-1791400000-d350d4a704221b947fca | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-4940000000-0e7b7b53bf836ee962a7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-059i-4900000000-ec326099d9ff20d07692 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|